Category: Intellectual Nourishment

PNAC in preterm infants: balancing nutrition, obstacles, and resources
Preterm infants often rely on parenteral nutrition (PN) to grow while the gut matures.1 In some cases, complications such as necrotizing enterocolitis (NEC) or congenital anomalies can lead to intestinal failure, making long-term PN essential.2
PN is life-sustaining but can also be expensive.3 A cross-sectional study of children with neonatal short bowel syndrome and intestinal failure (2004–2020) reported median initial hospitalizations of 150 days, with median costs exceeding $500,000.4
For many of these children, PN dependence continues well beyond the neonatal period, and growth remains a persistent challenge—about half of children with intestinal failure experience growth failure.5,6 A prolonged dependence on PN also increases the risk of complications such as parenteral nutrition–associated cholestasis (PNAC), often defined by a direct bilirubin ≥2 mg/dL.7
In severe cases, PNAC can progress to liver failure requiring transplantation and total medical costs exceeding more than $600,000 for commercially and Medicaid-insured patients within the first 3 months after surgery.8,9
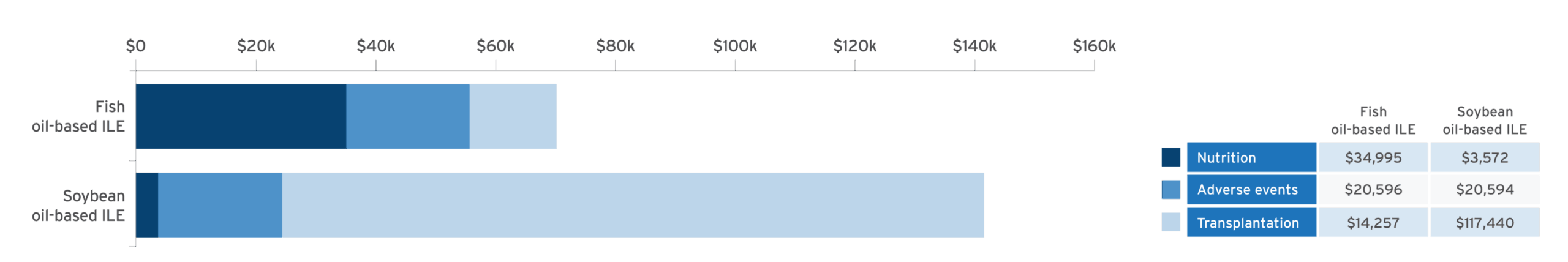
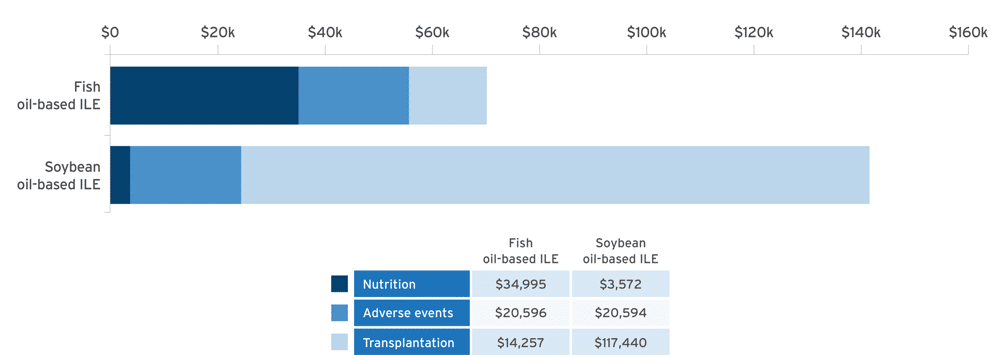
Economic modeling of estimated cost savings of fish oil lipid emulsion (FOLE) vs soybean oil lipid emulsion (SOLE) in PNAC10:
Adapted from Povero M, et al. JPEN J Parenter Enteral Nutr. 2025;49(2):180-188.10
A discrete event simulation model evaluated cost‐effectiveness by simulating clinical outcomes in 10,000 patients and estimating associated healthcare costs in pediatric patients with PNAC receiving PN with FOLE (1 g/kg/day) or SOLE (1.9 g/kg/day) over a time horizon of 6 years.10
- Results confirmed with probabilistic sensitivity analysis (95% confidence interval [CI])10
- Cost of Omegaven® (fish oil triglycerides) injectable emulsion, for intravenous use, was offset by the reduction in costs by avoidance of liver transplant10
Study limitations10
- Data were estimated using a historical cohort that received Intralipid® 20% (lipid injectable emulsion), for intravenous use, resulting in a difference of treatment eras
- Only the mortality rate was reported in the combined database, with no overall survival curves available
- Lack of longitudinal data for children with PNAC and precise data to estimate the cost of treatment for specific adverse events
Risk of Infections: Monitor for signs and symptoms; monitor laboratory parameters.11
Most common adverse drug reactions (>15%) are: vomiting, agitation, bradycardia, apnea and viral infection.11
Why this matters to healthcare systems and families
PN can be demanding—for patients, families, and the health system. Even small shifts in therapy can create ripple effects. While care must always be individualized, economic modeling studies offer perspective on how nutritional strategies may influence both financial outcomes and resources.
Could a fish oil lipid emulsion be right for your patients with PNAC?
About Omegaven® (fish oil triglycerides) injectable emulsion, for intravenous use
Omegaven is the only 100% fish oil lipid emulsion approved in the US for pediatric patients with PNAC. It provides omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).11 Omegaven may be used as part of a balanced PN regimen tailored to individual patient needs.
Please see the full Prescribing Information for Omegaven.
OMEGAVEN (fish oil triglycerides) injectable emulsion, for intravenous use
IMPORTANT SAFETY INFORMATION
These highlights do not include all the information needed to use OMEGAVEN safely and effectively. To learn more about OMEGAVEN for your child, talk to your child’s healthcare provider. OMEGAVEN is available by prescription only. The FDA-approved product labeling can be found at https://freseniuskabinutrition.com/OmegavenPI.
What is OMEGAVEN?
- A fish oil-based intravenous lipid emulsion that is a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
- Does not prevent PNAC.
- It has not been demonstrated that the clinical outcomes seen in pediatric patients are a result of the omega-6:omega-3 fatty acid ratio of the product.
- The hourly infusion rate should not exceed 1.5 mL/kg/hour
OMEGAVEN should not be received by patients who have:
- a known allergy to fish or egg protein or to any of the ingredients in OMEGAVEN.
- a severe bleeding disorder.
- abnormally high levels of lipid (triglycerides) in the blood.
What important safety information should I know about OMEGAVEN?
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
- The most common side effects, (>15%) include: vomiting, agitation, slower than normal heartbeat, interruption of breathing, and viral infection.
- These are not all the possible side effects associated with OMEGAVEN. Call your healthcare provider for medical advice regarding OMEGAVEN side effects. You are encouraged to report negative side effects of OMEGAVEN. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at https://freseniuskabinutrition.com/OmegavenPI.
Intralipid (lipid injectable emulsion) for intravenous use
IMPORTANT SAFETY INFORMATION
What is Intralipid?
- Indicated as a source of calories and essential fatty acids for adult and pediatric patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
Intralipid should not be received by patients who have:
- A known allergy to egg, soybean, or peanut, or any of the active ingredients or excipients in Intralipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
Intralipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly adhere to the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides: Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (≥5%) in adult patients include nausea, vomiting and fever and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase), and cholestasis (i.e., reducing or blocking the flow of bile).
These are not all the possible side effects associated with Intralipid. Call your healthcare provider for medical advice regarding Intralipid side effects. You are encouraged to report negative side effects of Intralipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling for Intralipid® 20% (lipid injectable emulsion), for intravenous use and Intralipid® 30% (lipid injectable emulsion), for intravenous use can be found at www.FreseniusKabiNutrition.com/Intralipid20PI and www.FreseniusKabiNutrition.com/Intralipid30PI.
Sources: 1. Robinson DT, Calkins KL, Chen Y, et al. Guidelines for parenteral nutrition in preterm infants: The American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2023;47(7):830-858. 2. Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. 2006;130(2 Suppl 1):S16-S28. 3. Worthington P, Balint J, Bechtold M, et al. When Is Parenteral Nutrition Appropriate?. JPEN J Parenter Enteral Nutr. 2017;41(3):324-377. 4. Raghu VK, Belaid S, Gutierrez S, et al. Social and Financial Costs of Neonatal Intestinal Failure. JAMA Netw Open. 2025;8(2):e2459548. Published 2025 Feb 3. 5. Goulet O, Ruemmele F, Lacaille F, Colomb V. Irreversible intestinal failure. J Pediatr Gastroenterol Nutr. 2004;38(3):250-269. 6. Pichler J, Horn V, Macdonald S, Hill S. Intestinal failure-associated liver disease in hospitalised children. Arch Dis Child. 2012;97(3):211-214. 7. Lapillonne A, Fidler Mis N, Goulet O, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Lipids. Clin Nutr. 2018;37(6 Pt B):2324-2336. 8. Gura K, Premkumar MH, Calkins KL, Puder M. Intravenous Fish Oil Monotherapy as a Source of Calories and Fatty Acids Promotes Age-Appropriate Growth in Pediatric Patients with Intestinal Failure-Associated Liver Disease. J Pediatr. 2020;219:98-105.e4. 9. Miloh T, Goldstein A, Howard R, et al. Costs of pediatric liver transplantation among commercially insured and Medicaid-insured patients with cholestasis in the US. Liver Transpl. 2023;29(7):735-744. 10. Povero M, Gura KM, Premkumar MH, Pradelli L, Puder M, Calkins KL. Fish oil lipid emulsion compared with soybean oil lipid emulsion in pediatric patients with parenteral nutrition-associated cholestasis: A cost-effectiveness study. JPEN J Parenter Enteral Nutr. 2025;49(2):180-188. 11. Omegaven Prescribing Information, Fresenius Kabi USA, LLC. 2025.
What can IV bags be made of?
A closer look at DEHP and PVC in intravenous (IV) bags
When it comes to parenteral nutrition (PN), the focus is rightly on what’s inside the bag—the nutrients that sustain patients who can’t meet their needs through food alone. But what’s containing these nutrients matters, too.
For decades, polyvinyl chloride (PVC) has been used to manufacture medical devices, IV bags, and tubing because it’s clear, strong, and flexible. To achieve that flexibility, PVC is commonly combined with plasticizers such as di(2-ethylhexyl) phthalate (DEHP).1
PVC used in medical devices often contains 30–40% DEHP by weight, and tubing can contain even higher concentrations.1
Although these materials have long supported modern medical practice, research suggests they may introduce considerations that go beyond function and convenience.
Understanding DEHP and PVC
DEHP isn’t chemically bound to PVC, which means it can migrate into the solutions it contains. In the clinical setting, that can result in measurable patient exposure—sometimes at levels significantly higher than those found in the general population.1
A critical review published in the American Journal of Industrial Medicine reported that DEHP and its metabolites have been associated in animal studies with effects on the liver, kidneys, lungs, and reproductive system. Developing infants and newborns appear especially sensitive to exposure during key periods of growth and development.1
While direct evidence in humans is limited, these findings have led many health authorities and hospitals to reassess the use of DEHP-containing PVC medical devices.1
Beyond the bedside
The conversation around PVC extends beyond patient exposure. Its full life cycle—from production to disposal—can generate environmental byproducts, including dioxins and vinyl chloride, that raise additional concerns. As sustainability goals become part of healthcare decision-making, demand for DEHP- and PVC-free alternatives continues to grow across systems and suppliers alike.1
A closer look at materials
Awareness of material composition is now part of broader patient-safety and sustainability efforts. For clinicians and pharmacists, that means evaluating not just what’s in a PN bag—but what the bag itself is made of.
To learn more about the materials and design considerations behind our IV and PN containers, visit this resource on innovative bag technology.
For more on clinical nutrition products available in containers not made with DEHP or PVC, visit FreseniusKabiNutrition.com.
Source: 1. Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of Di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am J Ind Med. 2001;39(1):100-111.

What a recent study reveals about intravenous lipid use in high-risk neonates requiring parenteral nutrition (and associated hepatic outcomes)
A recent randomized, controlled trial published in The Journal of Nutrition sheds light on how lipid injectable emulsion (ILE) choice may affect liver health, fatty acid status, and growth.
The study at a glance
A multicenter, randomized, double-blind, controlled trial enrolled 161 hospitalized neonates and infants across 14 sites in the US. All participants were expected to require at least 28 days of parenteral nutrition (PN) due to gastrointestinal conditions or surgical complications. The study compared a composite, fish oil-containing lipid emulsion (n=83) to a traditional soybean oil-based emulsion (n=78) as part of a full PN regimen.
-

Key details
Population: Term and preterm neonates and infants (≥24 weeks gestational age, ≥750 g birth weight) requiring ≥28 days of PN.
Intervention: The targeted lipid dose for PN was 3.0 g/kg body weight/d administered over 20 to 24 hours by central or peripheral vein infusion (mean lipid dose 2.0 ± 0.1 g/kg/d composite ILE, 2.6 ± 0.2 g/kg/d traditional soybean oil-based ILE).
Primary endpoint: Incidence of cholestasis, defined as conjugated bilirubin >2 mg/dL confirmed by a second sample 7 days later.
Secondary endpoints: Length of stay in the hospital and conjugated bilirubin concentrations in plasma.
-
Liver results
While the overall incidence of cholestasis was low across both groups—reflecting improvements in neonatal care—a few trends emerged:
- Patients who received the composite ILE had significantly lower conjugated bilirubin levels at the end of the initial treatment phase (P=0.006).
- No new cases of confirmed cholestasis were observed after day 28 in the composite ILE group.
- Patients receiving the composite ILE showed a trend toward a decreased risk of intestinal failure-associated liver disease (IFALD).
-

Fatty acid profiles
The study also measured changes in plasma and red blood cell polyunsaturated fatty acids (PUFAs). Findings include:
- Serum eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) levels increased at the end of the initial and extended treatment phases in patients receiving the composite ILE.
- No cases of essential fatty acid deficiency (EFAD) in either group until the end of the 84-day extended treatment phase based on the Holman index.*
*Insufficient data to determine the incidence of EFAD with SMOFlipid® >28 days. Cases of EFAD have been reported in adult and pediatric patients in the postmarketing period with SMOFlipid.
-

Clinical outcomes: growth, feeding, and length of stay
Despite more small-for-gestational-age infants in the composite group at baseline, both groups showed similar growth trajectories. Additional findings included:
- Comparable rates of transition to full enteral feeds.
- Overall median time to discharge from the hospital was 56.7 days for the composite ILE group and 66.4 days for the traditional soybean oil-based ILE group (secondary outcome, NS).
Why this matters
The data suggest that a composite ILE could be considered an alternative to a traditional soybean oil-based ILE in high-risk neonates.
Study limitations
There was a low incidence of cholestasis in both treatment groups compared to historical data. Additionally, there was a low completion rate (40%) predominantly due to earlier weaning from PN. The use of SMOFlipid in patients with established cholestasis was not assessed.
Read the full article here.
Abrams SA, Ernst KD, Weitkamp JH, et al. Safety and Efficacy of a Composite Lipid Emulsion with Fish Oil in Hospitalized Neonates and Infants Requiring Prolonged Parenteral Nutrition – A Randomized, Double-Blind, Multicenter, Controlled Trial. J Nutr. 2024;154(12):3615-3625.
This trial was funded by Fresenius Kabi Deutschland GmbH upon requirement of a postmarketing study from the United States Food and Drug Administration.
INDICATIONS AND USAGE
SMOFlipid is indicated in adult and pediatric patients, including term and preterm neonates, as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
IMPORTANT SAFETY INFORMATION
For intravenous infusion only into a central or peripheral vein. Use a non-vented non-DEHP 1.2 micron in-line filter set during administration. Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient. The recommended dose for adults and pediatrics is shown in Table 1. For information on age-appropriate infusion rate, see the full prescribing information. SMOFlipid Pharmacy Bulk Package is only indicated for use in pharmacy admixture programs for the preparation of three-in-one or total nutrition admixtures. Protect the admixed PN solution from light.
Table 1: Recommended Adult and Pediatric Dosage
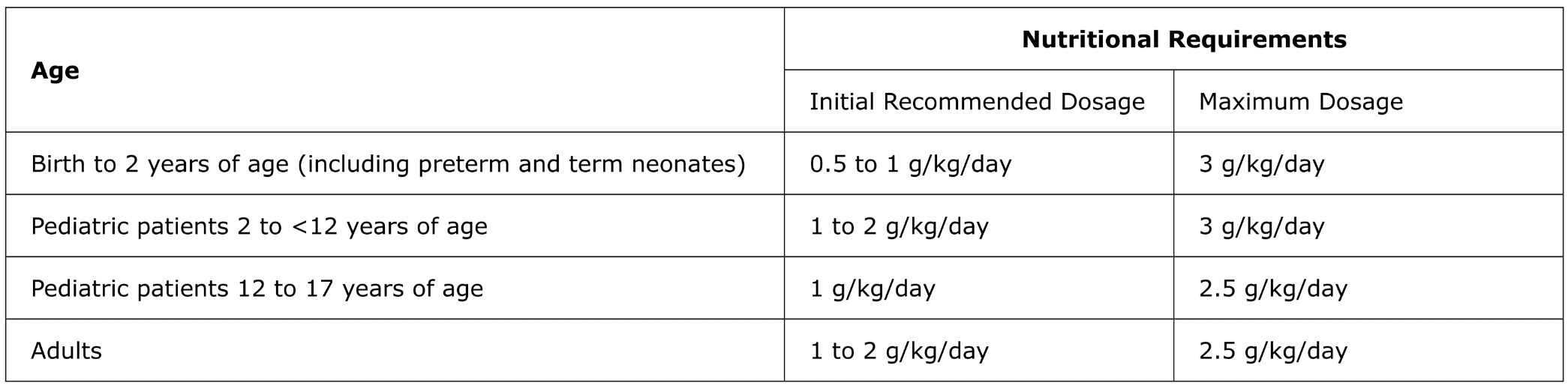
SMOFlipid is contraindicated in patients with known hypersensitivity to fish, egg, soybean, peanut, or any of the active or inactive ingredients, and severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL).
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported.
Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who received parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests; if abnormalities occur consider discontinuation or dosage reduction.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, Hypertriglyceridemia, and Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates.
Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting, and hyperglycemia. Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, and nosocomial infection.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use SMOFlipid safely and effectively. Please see full prescribing information for SMOFlipid (lipid injectable emulsion), for intravenous use at www.FreseniusKabiNutrition.com/SMOFlipidPI.
INDICATIONS AND USAGE
Intralipid is indicated as a source of calories and essential fatty acids for patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
IMPORTANT SAFETY INFORMATION
Intralipid 20% Pharmacy Bulk Package (lipid injectable emulsion), for intravenous use and Intralipid 30% Pharmacy Bulk Package (lipid injectable emulsion), for intravenous use are for admixing use only and are not intended for direct intravenous administration.
Intralipid 30% (lipid injectable emulsion) Pharmacy Bulk Package must be combined with other PN fluids. Diluting Intralipid 30% with an intravenous fluid such as normal saline or other diluent does not produce a dilution that is equivalent in composition to Intralipid 10% or 20% intravenous lipid emulsions. Therefore, diluents other than dextrose and amino acids should not be used to prepare admixtures for direct intravenous administration. When Intralipid 30% is diluted, strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.125 g/kg/hour for neonates and infants.
Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient. Protect the admixed PN solution from light. Use a 1.2 micron in-line filter during administration.
Dosage for Intralipid 20%

Intralipid is contraindicated in patients with:
- Known hypersensitivity to egg, soybean, or peanut, or any of the active ingredients or excipients
- Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglyceride > 1,000 mg/dL)
Risk of Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. When Intralipid 30% is diluted, strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.125 g/kg/hour for neonates and infants.
Risk of Parenteral Nutrition-Associated Liver Disease (PNALD): Increased risk in patients who receive PN for extended periods of time, especially preterm neonates. Monitor liver function tests; if abnormalities occur consider discontinuation or dosage reduction.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, and Hypertriglyceridemia: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates.
Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting, and pyrexia. Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, and cholestasis.
Vitamin K Antagonists (e.g., warfarin): Anticoagulant activity may be counteracted; increase monitoring of coagulation parameters.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use Intralipid safely and effectively. Please see full prescribing information, for intravenous use at www.FreseniusKabiNutrition.com/Intralipid20PI and www.FreseniusKabiNutrition.com/Intralipid30PI.

Undernourished and overlooked: addressing the silent epidemic of malnutrition
Malnutrition is a silent epidemic in hospitals—often overlooked, frequently underdiagnosed, and deeply consequential.1 Malnutrition, specifically undernutrition, is a lack of nutrients needed for a person’s health caused by impaired absorption, inadequate intake, increased nutrient needs, or altered nutrient transport and use.2
While the image of malnutrition can conjure thoughts of famine or food insecurity, it’s a clinical condition that affects an alarming number of patients with estimates suggesting that 1 in 3 are at risk.3 Some estimates say up to 60% of patients are malnourished.4 In critically ill patients, the risk can be even greater due to inflammation and altered metabolism.4,5
Left unaddressed, malnutrition may lead to increased length of hospital stay, higher healthcare costs, and greater morbidity and mortality rates.6-9 Through understanding how malnutrition is defined and diagnosed, clinicians can ethically and effectively address it.
Defining malnutrition
In adults, malnutrition can take several forms, including:
- Starvation-related malnutrition, such as from anorexia nervosa
- Chronic disease-related malnutrition, such as from organ failure or pancreatic cancer
- Acute disease or injury-related malnutrition, such as from burns, trauma, or major infection10
During illness, nutrition intake is critical in supporting body functions involved in recovery. In fact, up to 80% of intensive care unit (ICU) patients may be malnourished, which increases ICU length of stay (LOS), hospital LOS, increases readmission rates to the hospital, and have up to 6.5x more cost compared to general ward patients.5
Malnutrition diagnosis: criteria and importance
Currently, the Academy of Nutrition and Dietetics (AND) and American Society for Parenteral and Enteral Nutrition (ASPEN) Indicators to Diagnose Malnutrition (AAIM) and the Global Leadership Initiative on Malnutrition (GLIM) both have distinct frameworks for diagnosing malnutrition.
AAIM assesses factors such as weight loss, energy intake, fat and muscle loss, edema, and hand grip strength. It’s been shown to have predictive validity for patient outcomes. GLIM is designed as a global consensus on criteria, allowing for global comparisons of malnutrition prevalence, treatments, and outcomes.10
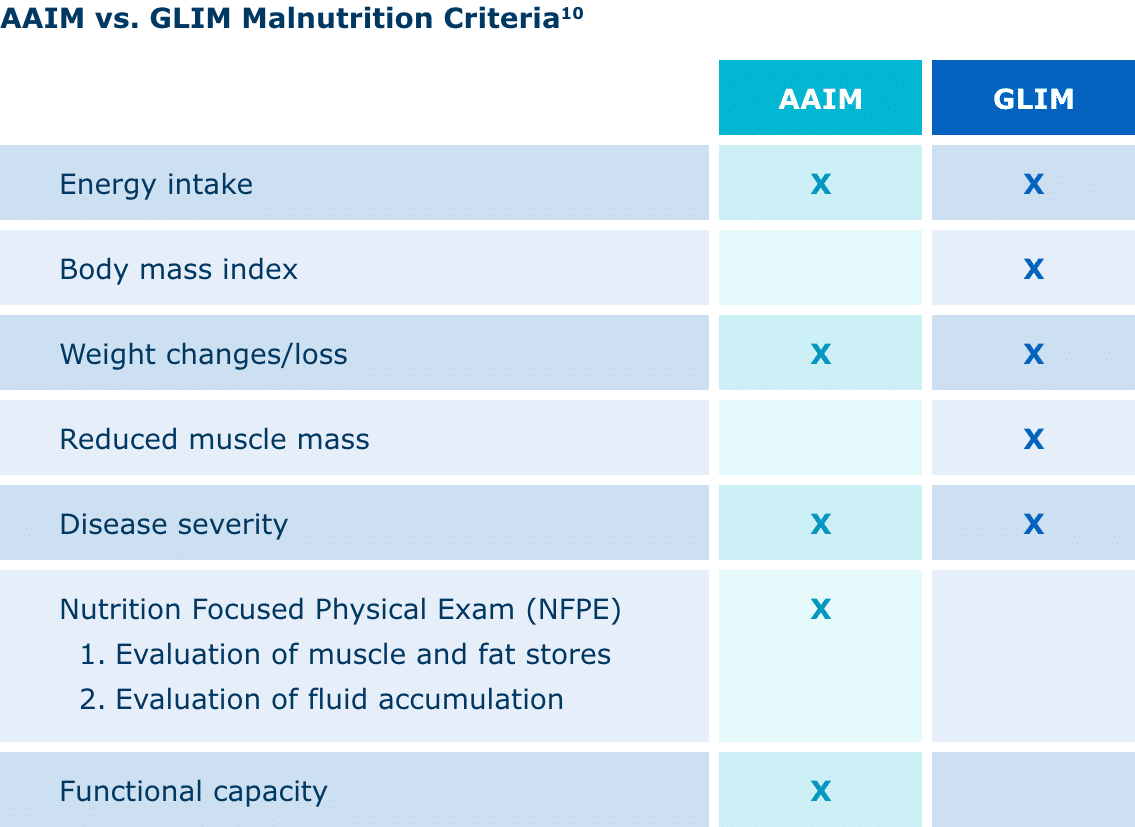
Diagnosing and treating malnutrition in a timely and effective manner is directly related to clinical outcomes. Malnutrition can increase length of stay, hospital costs, and readmission rates. It can also contribute to poor wound healing and recovery.5
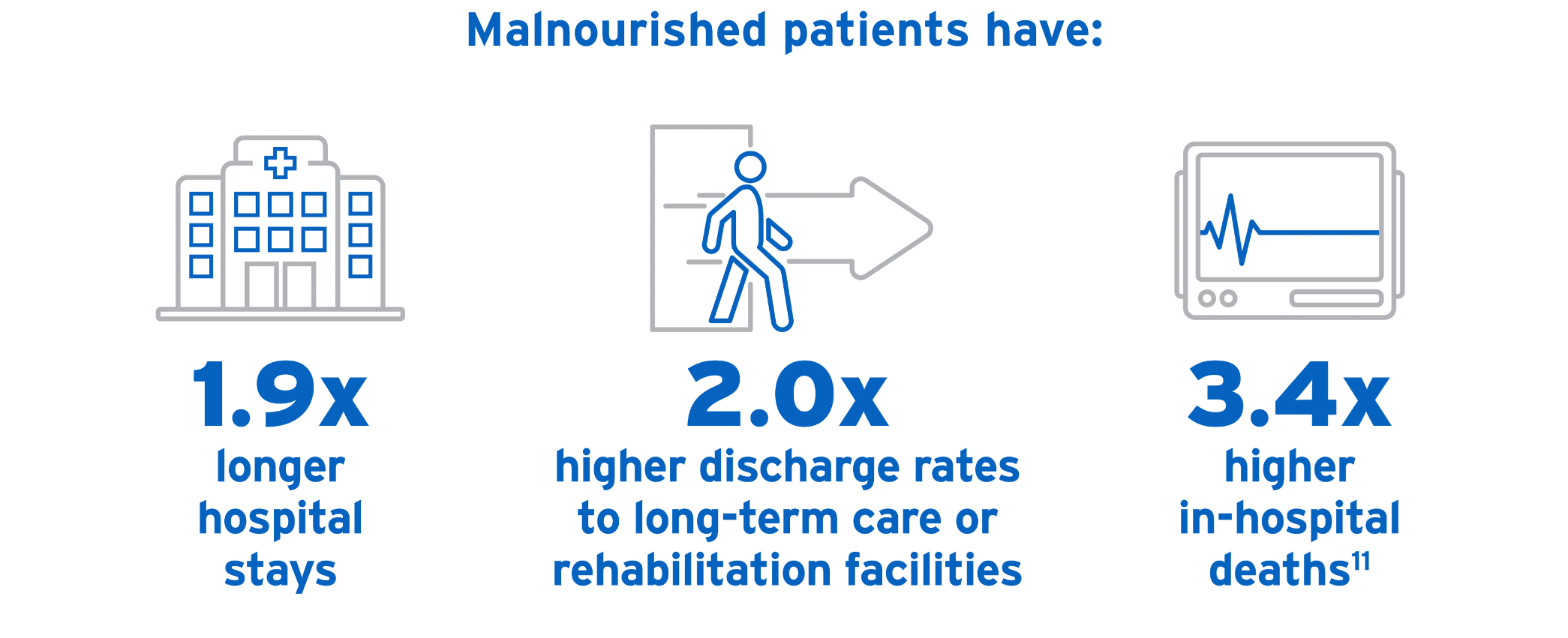
Because malnutrition often goes unnoticed, a universal set of diagnostic criteria is critical. Not only will this help healthcare providers recognize malnutrition, contributing to more accurate estimates of its prevalence, but it will also help guide best practices and inform expected outcomes. Even more, it will help predict and mitigate the financial burdens of malnutrition’s prevention and treatment.4
Using parenteral nutrition to address nutritional gaps in hospitalized patients
Parenteral nutrition or PN has proven efficacy in helping hospitalized patients meet nutritional goals.12 After a full evaluation and ruling out the feasibility of enteral nutrition, PN should be used for patients who are malnourished or at risk of malnourishment.13
ASPEN’s guide to PN11:
- Don’t use PN based only on medical diagnosis or disease state.
- Before initiating PN, conduct a complete evaluation of the feasibility of enteral nutrition (EN), using medical history, physical examination, and diagnostic evaluations.
- After ruling out EN, use PN in patients who are malnourished or at risk of malnourishment.
- Begin PN:
- After 7 days for well-nourished, stable adult patients who haven’t been able to receive 50% or more oral or enteral nutrients.
- Within 3 to 5 days for patients who are nutritionally at risk and aren’t likely to achieve desired oral intake or EN.
- As soon as is feasible for patients with baseline moderate or severe malnutrition and insufficient or impossible oral intake or PN.
- Delay PN in patients who have severe metabolic instability until they are improved.
As part of a nutrition care plan, including PN, multi-oils and alternative lipid injectable emulsions (ILEs) should be considered.
PN is not without risks, with the primary concern being infection of the bloodstream.14
Still, PN is a critical tool in nutritional care for malnourished or high-risk patients, and the benefits far outweigh the risks in giving patients a chance to heal and recover. For many patients, PN can be lifesaving.
Moving forward in clinical nutrition
Malnutrition is prevalent and serious—but it can be treatable. Clinicians must assess, diagnose, and act quickly, using standardized criteria and following best practice guidelines to improve the nutritional status and outcomes of patients.
Sources: 1. Guenter P, Blackmer A, Malone A, et al. Update on use of enteral and parenteral nutrition in hospitalized patients with a diagnosis of malnutrition in the United States. Nutr Clin Pract. 2022;37(1):94-101. 2. Galang M, Bury C, Pogatschnik C, Dowhan L. Chapter 11: Malnutrition Screening and Assessment. In: Chan LN, ed. ASPEN Adult Nutrition Support Core Curriculum. 4th ed. American Society for Parenteral and Enteral Nutrition; 2025:257-258. 3. Sauer AC, Goates S, Malone A, et al. Prevalence of Malnutrition Risk and the Impact of Nutrition Risk on Hospital Outcomes: Results From nutritionDay in the U.S.. JPEN J Parenter Enteral Nutr. 2019;43(7):918-926. https://doi.org/10.1002/jpen.1499 4. White JV, Guenter P, Jensen G, et al. Consensus Statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2012;36(3):275-283. https://doi.org/10.1177/0148607112440285 5. Why nutrition is important: Critically ill patient. ASPEN Website. 2020. Accessed June 3, 2025. https://nutritioncare.org/wp-content/uploads/2024/12/Critically-Ill-Patient-Why-Nutrition-is-Important.pdf 6. National Alliance for Infusion Therapy and the American Society for Parenteral and Enteral Nutrition Public Policy Committee and Board of Directors. Disease-related malnutrition and enteral nutrition therapy: a significant problem with a cost-effective solution. Nutr Clin Pract. 2010;25(5):548-554. 7. Jensen GL, Bistrian B, Roubenoff R, Heimburger DC. Malnutrition syndromes: a conundrum vs continuum. JPEN J Parenter Enteral Nutr. 2009;33(6):710-716. 8. Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. JPEN J Parenter Enteral Nutr. 2010;34(2):156-159. 9. Jensen GL. Inflammation as the key interface of the medical and nutrition universes: a provocative examination of the future of clinical nutrition and medicine. JPEN J Parenter Enteral Nutr. 2006;30(5):453-463. 10. Key nutrition screening, assessment, and malnutrition diagnostic processes and tools for adults. ASPEN Website. July 30, 2024. Accessed June 3, 2025. https://nutritioncare.org/wp-content/uploads/2024/12/Nutrition-Screen-Assess-Diagnose-Adults.pdf 11. Parenteral nutrition therapy for malnutrition. ASPEN Website. August 29, 2022. Accessed June 3, 2025. https://nutritioncare.org/wp-content/uploads/2024/12/Malnutrition-PN-Indication.pdf 12. Ayers P, Bobo ES, Hunt RT, Mays AA, Worthington PH, eds. ASPEN Parenteral Nutrition Handbook. 3rd ed. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition; 2020:20-21,37. 13. Worthington P, Balint J, Bechtold M, et al. When Is Parenteral Nutrition Appropriate?. JPEN J Parenter Enteral Nutr. 2017;41(3):324-377. 14. Ayers P, Adams S, Boullata J, et al. A.S.P.E.N. parenteral nutrition safety consensus recommendations. JPEN J Parenter Enteral Nutr. 2014;38(3):296-333.

4 oils in 1 lipid emulsion: see the difference
As the US market leader in lipid injectable emulsions (ILEs),1 we’re proud to offer SMOFlipid® (lipid injectable emulsion), for intravenous use, as an option for parenteral nutrition (PN). With its unique blend, SMOFlipid demonstrates our commitment to providing an alternative to soybean oil ILEs, helping to support the nutrition needs of patients at any age.2
Designed with more oils for a balanced fatty acid profile
A source of calories and essential fatty acids (EFAs) for PN, SMOFlipid nourishes patients—from stable to critically and chronically ill—with a one-of-a-kind blend of 4 oil sources.2
SMOFlipid’s unique blend of 4 oils allows clinicians to provide daily lipids for patients requiring PN.2
Indicated for daily lipid dosing2
Daily lipids can be a part of PN because they provide EFAs and are an alternative to dextrose as a sole energy source, which can help minimize the complications of excessive dextrose administration, including hepatic steatosis, respiratory insufficiency, hyperglycemia-induced compromised immune function, metabolic stress, and fever.5,6


Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient.2
Do not exceed the maximum infusion rate of 0.5 mL/kg/hour in adults and 0.75 mL/kg/hour in pediatrics.2
Please refer to the Full Prescribing Information for complete dosing guidance.
Contains EFAs to support EFA needs2
SMOFlipid contains linoleic acid (LA) and alpha-linolenic acid (ALA), which are precursors to long-chain polyunsaturated fatty acids (LCPUFAs) that help prevent essential fatty acid deficiency (EFAD)2,8,9:
- LA is an omega-6 FA and precursor to arachidonic acid (ARA)
- ALA is an omega-3 FA and precursor to EPA and DHA
Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters.2
Proven to have a well-established safety and tolerability profile2
The US Food and Drug Administration (FDA) approved SMOFlipid for adults in 2016; six years later in 2022, SMOFlipid received approval for use in pediatric patients, including term and preterm neonates.2
There has been a recent publication from a post-marketing study.10 Read the full article.
SMOFlipid is a trusted lipid for the top 10 children’s hospitals*†
*Data on file 3/1/25.
†As reported by US News & World Report: https://health.usnews.com/best-hospitals/pediatric-rankings
SMOFlipid has been extensively researched, including in studies in more than 175 adult patients in 3 clinical trials and in more than 170 pediatric patients in 4 randomized, active-controlled, double-blind, parallel-group controlled clinical trials.2
In addition, Fresenius Kabi has developed comprehensive admixture stability and Y-site drug compatibility reference guides to help ensure the stability, compatibility, and integrity of admixtures for adult and pediatric patients. Check them out here.
Discover the one and only SMOFlipid
SMOFlipid is globally recognized, with approval in more than 75 countries. This unique blend of 4 oil sources provides a source of calories and EFAs to help nourish adult and pediatric patients requiring PN.2
Learn more about SMOFlipid:
SMOFlipid® (lipid injectable emulsion, USP), for intravenous use IMPORTANT SAFETY INFORMATION
What is SMOFlipid?
- Indicated in adult and pediatric patients as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
- The hourly infusion rate in pediatrics should not exceed 0.75 mL/kg/hour and 0.5 mL/kg/hour in adults.
SMOFlipid should not be received by patients who have:
- A known allergy to fish, egg, soybean, or peanut, or to any of the active or inactive ingredients in SMOFlipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
SMOFlipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (>1%) in adult patients include nausea, vomiting, and high levels of glucose in the blood and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase) and hospital- acquired infections.
These are not all the possible side effects associated with SMOFlipid. Call your healthcare provider for medical advice regarding SMOFlipid side effects. You are encouraged to report negative side effects of SMOFlipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at https://freseniuskabinutrition.com/SMOFlipidPI.
Sources: 1. Data on File; 3/1/25; calculation includes: all ILEs approved in the US. 2. SMOFlipid Prescribing Information, Fresenius Kabi USA, LLC. 2023. 3. Kalish BT, Fallon EM, Puder M. A tutorial on fatty acid biology. JPEN J Parenter Enteral Nutr. 2012;36(4):380-388. 4. Deckelbaum RJ, Hamilton JA, Moser A, et al. Medium-chain versus long-chain triacylglycerol emulsion hydrolysis by lipoprotein lipase and hepatic lipase: implications for the mechanisms of lipase action. Biochemistry. 1990;29(5):1136-1142. 5. Vanek VW, Seidner DL, Allen P, et al. A.S.P.E.N. position paper: Clinical role for alternative intravenous fat emulsions. Nutr Clin Pract. 2012;27(2):150-192. 6. Calder PC, Jensen GL, Koletzko BV, Singer P, Wanten GJ. Lipid emulsions in parenteral nutrition of intensive care patients: current thinking and future directions. Intensive Care Med. 2010;36(5):735-749. 7. ASPEN Lipid Injectable Emulsion Safety Recommendations for Adult Patients. ASPEN website. 2021. Accessed March 4, 2025. https://nutritioncare.org/wp-content/uploads/2024/12/ILE-Safety-Recommendations-Adult.pdf 8. Agostoni C. Role of long-chain polyunsaturated fatty acids in the first year of life. J Pediatr Gastroenterol Nutr. 2008;47 Suppl 2:S41-S44. 9. Fell GL, Nandivada P, Gura KM, Puder M. Intravenous Lipid Emulsions in Parenteral Nutrition. Adv Nutr. 2015;6(5):600-610. Published 2015 Sep 15. 10. Abrams SA, Ernst KD, Weitkamp JH, et al. Safety and Efficacy of a Composite Lipid Emulsion with Fish Oil in Hospitalized Neonates and Infants Requiring Prolonged Parenteral Nutrition – A Randomized, Double-Blind, Multicenter, Controlled Trial. J Nutr. 2024;154(12):3615-3625.

Multi-chamber bags (MCBs): streamlining parenteral nutrition (PN)
MCB products can be options for many adult patients who require PN. In fact, these innovations are widely used in Europe.1 MCB-PN products are becoming more popular in the US, possibly because they help address the need for compounded clinical nutrition alternatives during PN product shortages and offer the potential to reduce pharmacy staff time and costs.*2-6 Let’s take a look at what MCB-PN products are, explore their features, review safety concerns, and more.
A clinical guidelines recommendation from the American Society for Parenteral and Enteral Nutrition (ASPEN) states, “We suggest that commercially available premade multichambered PN formulations be considered as an available option for patients alongside compounded (customized or standardized) PN formulations to best meet an organization’s patient needs.”6
What is MCB-PN?
MCB-PN products are available as 2- and 3-chamber bags, each designed with a unique formulation to help support the various nutrition needs of many patients requiring PN.7-9
![]()
A 2-chamber bag (2CB) features 2 separate compartments—one for dextrose and one for amino acids. Lipid injectable emulsions may be added to the bag, and formulations are available with or without electrolytes.7
![]()
A 3-chamber bag (3CB) features 3 separate compartments for dextrose, amino acids with electrolytes, and a soybean oil-based lipid emulsion. Currently, there are no electrolyte-free versions available in the US.8,9
However, their use may not be appropriate for patients with high gastrointestinal tract output, severe electrolyte abnormalities, or in those who require more amino acids and calories that cannot be provided with MCB-PN alone.*10 These patients may require customized formulations to help them meet their unique nutrition requirements.

Fresenius Kabi’s 3CB formulations—Kabiven® (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use, and Perikabiven® (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use—help clinicians efficiently deliver 3 macronutrients (dextrose, protein, and lipids) plus electrolytes in volumes and concentrations that meet the needs of many adult patients by reducing waste.5,8,9 These products are the first and only of their kind available for adults in the US.8,9
Please see Important Safety Information below.
What are the features of Fresenius Kabi’s 3CB-PN products?
3CB-PN products require fewer compounding steps compared with manual compounding.2 They are also designed for convenience by mixing nutrients in a single bag, facilitating standardized PN processes.2 The features of our very own 3CB products, Kabiven and Perikabiven, may help:
![]()
Simplify setup: No need to piggyback or add lipids manually
![]()
Streamline delivery: All-in-one formulation delivers macronutrients and electrolytes simultaneously, eliminating the need for Y-site infusion
![]()
Add flexibility: Two formulations to help meet adult patients’ unique needs — Kabiven for central PN and Perikabiven for peripheral or central PN8,9
![]()
Save time and costs: 3CBs may be associated with reduced pharmacy staff time, workload, and costs compared with hospital-compounded bags (HCBs)5
A time and motion study evaluated time, labor, and cost savings of 3CBs compared with HCBs.
In a multicenter, prospective, time and motion study evaluating PN delivery systems, the 3CB delivery system was associated with a 62% reduction in pharmacy staff time and workload as well as a 37% reduction in costs compared with HCBs (representing PN prepared with automated compounding devices). The cost per bag included labor, PN products, medical consumables, and equipment. One hundred thirty-six PN prescriptions were prepared during the study (66 for 3CBs and 70 for HCBs). The clinical efficacy of 3CBs versus compounded PN was not evaluated.5
Are there any safety concerns associated with MCB-PN?
Although MCB-PN requires fewer compounding steps, there are safety issues to keep in mind once the bags are activated. For example, the length of stability and storage conditions vary, depending on the absence or addition of additives.8,9,11,12
While MCB-PN can be an option for many patients requiring PN, it’s important to consider several factors to determine if these products could be a good fit for your facility.
Check out this ASPEN practice tool to learn more about the use of MCB-PN.
As the global leader in 3-chamber bags† and US market leader in lipid injectable emulsions,13 Fresenius Kabi is proud to offer Kabiven and Perikabiven as options for PN.
See how Kabiven’s and Perikabiven’s unique designs help streamline the delivery of nutrition therapy to adult patients and may simplify calculations, prescription writing, compounding, and administration: www.FreseniusKabiNutrition.com/products/kabiven-perikabiven/
KABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use PERIKABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
IMPORTANT SAFETY INFORMATION
What is Kabiven and Perikabiven?
- Indicated in adult patients as a source of calories, protein, electrolytes and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adults.
- Do not exceed the recommended maximum infusion rate of 2.6 mL/kg/hour for Kabiven and 3.7 mL/kg/hour for Perikabiven.
Limitations of Use
Neither Kabiven nor Perikabiven is recommended in pediatric patients less than 2 years old because the fixed amount of the formulations do not meet nutritional needs in this age group.
Do not use Kabiven or Perikabiven in patients who have:
- Simultaneous treatment with ceftriaxone in neonates (28 days of age or younger)
- Known allergy to egg, soybean, peanut or any of the active or inactive ingredients
- Abnormally high levels of lipid (triglycerides) in the blood (with serum triglyceride concentration >1,000 g/dL)
- Inborn errors of amino acid metabolism (a genetic defect in protein metabolism)
- Cardiopulmonary instability (inability for the heart and lungs to function right)
- Hemophagocytic syndrome (a disorder of the immune system)
Kabiven and Perikabiven may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who receive parenteral nutrition for greater than 2 weeks. Your healthcare provider will monitor liver tests.
- Pulmonary Embolism (a blockage in a blood vessel in the lung) and Respiratory Distress (increased breathing rate, bluish skin color changes, wheezing) due to Pulmonary Vascular Precipitates (solid substance in the blood vessel of the lungs): If signs of lung issues occur, stop the infusion and start a medical evaluation.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction
- Precipitation (solid substance in the blood vessel) with Ceftriaxone: Do not administer ceftriaxone simultaneously with Kabiven or Perikabiven via a Y-site.
- Infection, fat overload, hyperglycemia (high blood sugar) and refeeding syndrome: Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels
The most common adverse reactions for Kabiven (≥3%) are nausea, fever, high blood pressure, vomiting, decreased blood hemoglobin, decreased blood total protein, low blood potassium, and increased gamma glutamyltransferase (a liver enzyme). The most common adverse reactions for Perikabiven (≥3%) are high blood sugar, low blood potassium, fever and increased blood lipids.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Tell your doctor if you are taking coumarin and coumarin derivatives, including warfarin: the drug activity may be lessened and your healthcare provider will monitor your blood.
These are not all the possible side effects associated with Kabiven and Perikabiven. Call your healthcare provider for medical advice regarding Kabiven and Perikabiven side effects. You are encouraged to report negative side effects of Kabiven and Perikabiven. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.FreseniusKabiNutrition.com/KabivenPI and www.FreseniusKabiNutrition.com/PerikabivenPI.
*Andrew Mays is an employee of Fresenius Kabi as of January 8, 2024.
†MIDAS Database MAR 2024.
Sources: 1. Ayers P, Berger MM, Berlana D, et al. Expert consensus statements and summary of proceedings from the International Safety and Quality of Parenteral Nutrition Summit. Am J Health Syst Pharm. 2024;81(Supplement_3):S75-S88. 2. Multi-Chamber Bag Parenteral Nutrition: Indications, Product Availability, and Patient Safety Practice Tool. ASPEN Website. January 18, 2024. Accessed March 10, 2025. https://nutritioncare.org/wp-content/uploads/2024/12/MCB-PN-Practice-Tool.pdf 3. Mays A, Ayers P, Murphy MK. Address PN Shortages with MCB-PN. Pharm Purchasing Prod. 2022;19(11):18. 4. Nystrom EM, Bergquist WJ, Wieruszewski PM, McMahon MM, Barreto EF. Parenteral Nutrition Drug Shortages: A Single-Center Experience With Rapid Process Change. JPEN J Parenter Enteral Nutr. 2019;43(5):583-590. 5. Cogle SV, Martindale RG, Ramos M, et al. Multicenter Prospective Evaluation of Parenteral Nutrition Preparation Time and Resource Utilization: 3-Chamber Bags Compared With Hospital Pharmacy-Compounded Bags. JPEN J Parenter Enteral Nutr. 2021;45(7):1552-1558. 6. Boullata JI, Gilbert K, Sacks G, et al. A.S.P.E.N. clinical guidelines: parenteral nutrition ordering, order review, compounding, labeling, and dispensing. JPEN J Parenter Enteral Nutr. 2014;38(3):334-377. 7. Baxter Hospital Products. Baxter website. Accessed December 19, 2024. https://ushospitalproducts.baxter.com/clinimix-amino-acids-dextrose-injections-0 8. Kabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 9. Perikabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 10. Mays A, Ayers P. Patient Selection and Safety Considerations for Multi-Chamber Bag Parenteral Nutrition. Pharmacy Practice News. May 16, 2023. Accessed January 16, 2025. https://www.pharmacypracticenews.com/Clinical/Article/05-23/Patient-Selection-and-Safety-Considerations-for-Multi-Chamber-Bag-Parenteral-Nutrition/70258 11. Clinimix Prescribing Information, Baxter Healthcare Corporation. 2021. 12. Clinimix E Prescribing Information, Baxter Healthcare Corporation. 2021. 13. Data on File; 1/1/25; calculation includes: all ILEs approved in the US.

Pediatric parenteral nutrition (PN): more options for more young patients
PN has been administered to children for more than 40 years.1,2 This form of nutrition support can be lifesaving for many pediatric patients who are unable to receive adequate nutrition via the enteral route, which is often due to a gastrointestinal disease or condition such as intestinal failure, short bowel syndrome, or a bowel obstruction.3
As the US market leader in lipid injectable emulsions (ILEs),4 we provide clinicians and their pediatric patients with a variety of PN choices. From a mixed-oil blend to an omega-3-rich emulsion to a 100% soybean oil-based formulation, Fresenius Kabi offers more options for unique pediatric nutrition needs.
Explore the 4-oil difference
SMOFlipid is our proprietary ILE that helped us bring the benefits of alternative lipid emulsions to market. Designed to provide a source of calories and essential fatty acids (EFAs), SMOFlipid nourishes pediatric patients with a one-of-a-kind blend of 4 oil sources5:
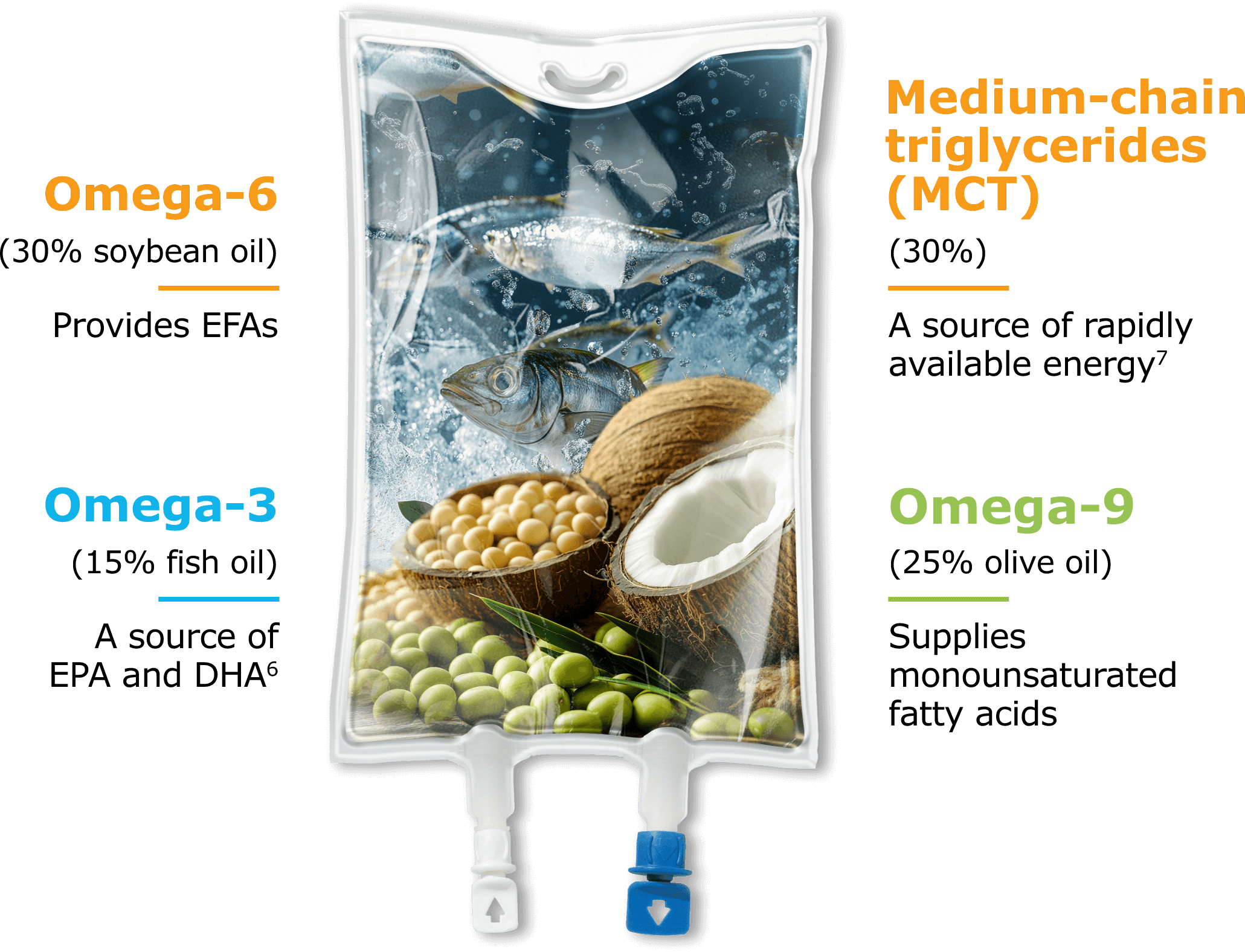
PN-associated cholestasis (PNAC) developed less frequently in pediatric patients fed a 4-oil ILE versus a 100% soybean oil (SO) ILE.5
In a randomized clinical trial among neonates and infants expected to be treated with PN for at least 28 days, PNAC, a precursor to PN-associated liver disease (PNALD), developed less frequently in SMOFlipid-treated patients than in 100% SO lipid emulsion-treated patients.5
Pediatric Study 1 also compared the incidence of PNAC (DBIL >2 mg/dL with a second confirmed DBIL >2mg/dL at least 7 days later) in both groups5:
- PNAC mostly occurred in patients who received treatment for more than 28 days
- 2.4% (2/83) of SMOFlipid-treated patients developed PNAC
- 11.5% (9/78) of SO lipid emulsion-treated patients developed PNAC
One company-sponsored trial showed equivalent total bilirubin and direct bilirubin levels comparing SMOFlipid with a soybean-based ILE.
*Data on file 11/1/24.
Nurture with omega-3-rich PN
Premature infants with PNAC may require specific nutrients. Rich in omega-3 fatty acids, Omegaven is the first and only 100% fish oil lipid emulsion in the US for pediatric patients with PNAC, and it has been shown to help achieve age-appropriate growth.8
Fresenius Kabi is committed to bringing more to pediatric PN. Thank you for partnering with us to nourish tiny patients who require clinical nutrition support.
INDICATIONS AND USAGE
Intralipid® 20% (lipid injectable emulsion) for intravenous use, Intralipid 30% (lipid injectable emulsion) for intravenous use Pharmacy Bulk Package, SMOFlipid 20%, (lipid injectable emulsion), for intravenous use
Intralipid and SMOFlipid are indicated as a source of calories and essential fatty acids for adult and pediatric patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
Pharmacy Bulk Packages are for admixing only and are not intended for direct intravenous infusion.
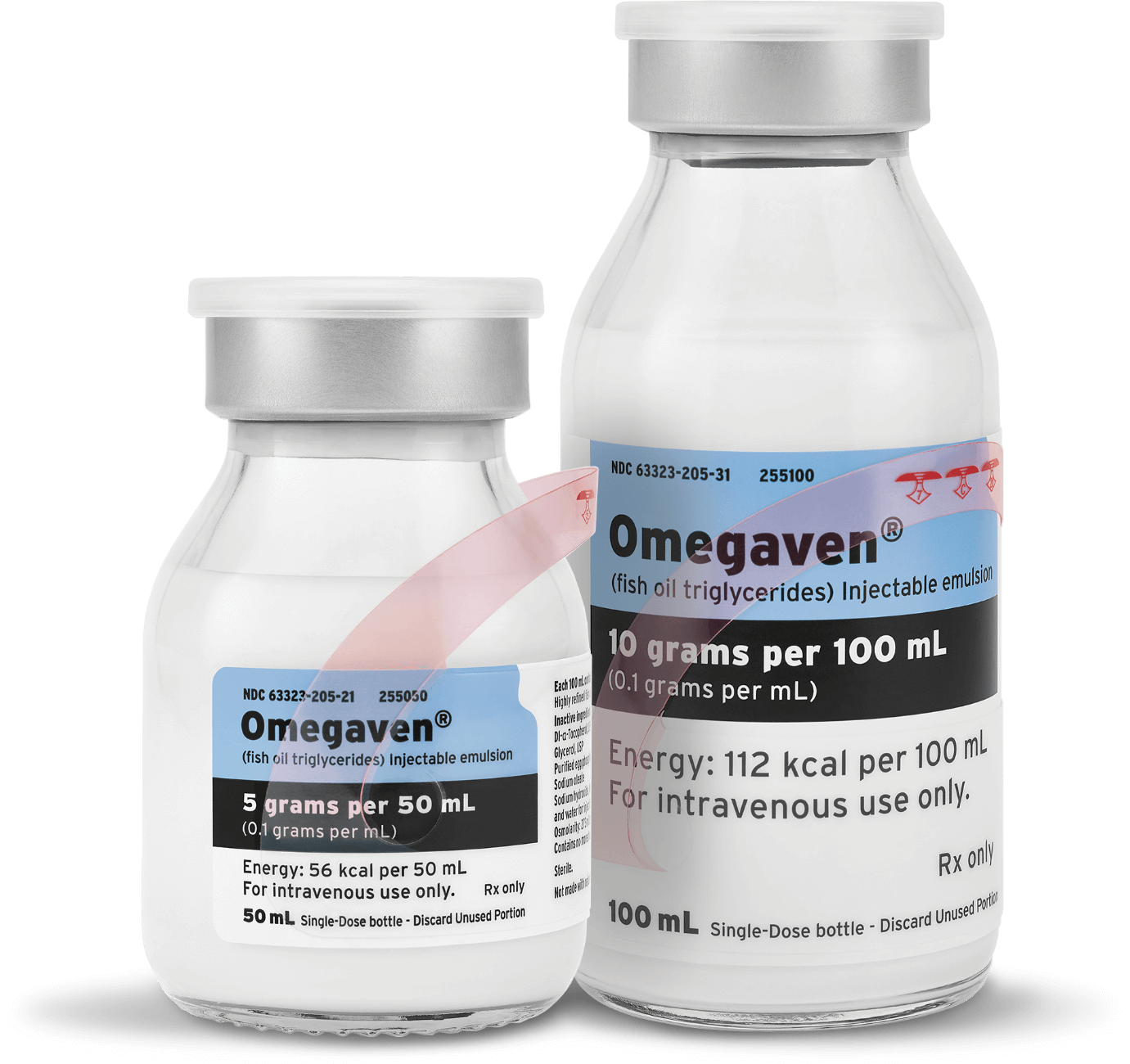
Omegaven (fish oil triglycerides) injectable emulsion, for intravenous use
Omegaven is indicated as a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
Limitations of Use: Omegaven is not indicated for the prevention of PNAC. It has not been demonstrated that Omegaven prevents PNAC in PN-dependent patients. It has not been demonstrated that the clinical outcomes observed in patients treated with Omegaven are a result of the omega-6:omega-3 fatty acid ratio of the product.
IMPORTANT SAFETY INFORMATION
Protect the PN admixture from light. Use a non-DEHP infusion set and 1.2 micron-inline filter during administration. Prior to administration, correct severe fluid and electrolyte disorders. Recommended dosage depends on age, energy expenditure, clinical status, body weight, ability to metabolize and eliminate lipids and additional energy given to the patient. The recommended SMOFlipid and Intralipid dose for adults and pediatrics is shown in Table 1 and recommended Omegaven dosing is shown in Table 2. For information on age-appropriate infusion rates and maximum infusion rates, see the full prescribing information. For Omegaven, initiate dosing in PN-dependent pediatric patients as soon as direct or conjugated bilirubin levels are 2 mg/dL or greater. Administer Omegaven until direct or conjugated bilirubin levels are less than 2 mg/dL or until the patient no longer requires PN.
Contraindications include the following: known sensitivity to the active or inactive ingredients; severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1000 mg/dL); severe hemorrhagic disorders (Omegaven).
Table 1: Recommended Dosage Intralipid 20% and SMOFlipid 20%
| Age | Nutritional Requirements | |
|---|---|---|
| Initial Recommended Dosage | Maximum Dosage | |
| Birth to 2 years of age (including preterm and term neonates) |
SMOFlipid: 0.5 to 1 g/kg/day Intralipid: 0.5 g/kg/day |
3 g/kg/day |
| Pediatric patients 2 to <12 years of age | 1 to 2 g/kg/day | SMOFlipid: 3 g/kg/day Intralipid: 2.5 g/kg/day |
| Pediatric patients 12 to 17 years of age | 1 g/kg/day | SMOFlipid: 2.5 g/kg/day Intralipid: 2 g/kg/day |
| Adults | SMOFlipid: 1 to 2 g/kg/day Intralipid: 1 g/kg/day (stable); ≤ g/kg/day (critically ill) |
2.5 g/kg/day |
Table 2: Recommended Omegaven and Infusion Rate
| Nutritional Requirements | Direct Infusion Rate | |
|---|---|---|
| Recommended Initial Dosage and Maximum Dosage |
Initial | Maximum |
| 1 g/kg/day; this is also the maximum daily dose |
0.2 mL/kg/hour for the first 15 to 30 minutes; gradually increase to the required rate after 30 minutes |
1.5 mL/kg/hour |
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. Strictly adhere to the hourly infusion rate. Carefully monitor the infant’s ability to eliminate the infused lipids from the circulation (e.g., measure serum triglycerides and/or plasma free fatty acid levels). If signs of poor clearance of lipids from the circulation occur, stop the infusion and initiate a medical evaluation. When Intralipid 30% is diluted to 20%, strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.125 g/kg/hour for neonates and infants.
Parenteral Nutrition-Associated Liver Disease (PNALD): Increased risk in patients who receive PN for extended periods of time, especially preterm neonates. Monitor liver function tests; if abnormalities occur consider discontinuation or dosage reduction (Intralipid and SMOFlipid).
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, and Hypertriglyceridemia and Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates
Monitoring and Laboratory Tests: Routine laboratory monitoring is recommended, including monitoring for essential fatty acid deficiency.
Intralipid and SMOFlipid: Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting (SMOFlipid and Intralipid), pyrexia (Intralipid) and hyperglycemia (SMOFlipid). Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, cholestasis (Intralipid), and nosocomial infection (SMOFlipid).
Intralipid and SMOFlipid: Vitamin K Antagonists (e.g., warfarin): Anticoagulant activity may be counteracted; increase monitoring of coagulation parameters.
Omegaven: The most common adverse drug reactions (>15%) are: vomiting, agitation, bradycardia, apnea and viral infection.
Omegaven: Antiplatelet Agents and Anticoagulants: Prolonged bleeding time has been reported in patients taking antiplatelet agents or anticoagulants and oral omega-3 fatty acids. Periodically monitor bleeding time in patients receiving Omegaven and concomitant antiplatelet agents or anticoagulants.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use SMOFlipid, Intralipid and Omegaven safely and effectively. Please see full prescribing information, for SMOFlipid, Intralipid and Omegaven at www.FreseniusKabiNutrition.com/SMOFlipidPI, www.FreseniusKabiNutrition.com/Intralipid20PI, www.FreseniusKabiNutrition.com/Intralipid30PI, and www.FreseniusKabiNutrition.com/OmegavenPI.
Sources: 1. Jimenez L, Mehta NM, Duggan CP. Timing of the initiation of parenteral nutrition in critically ill children. Curr Opin Clin Nutr Metab Care. 2017;20(3):227-231. 2. Wilmore DW, Dudrick SJ. Growth and development of an infant receiving all nutrients exclusively by vein. JAMA. 1968;203(10):860-864. 3. What Is Parenteral Nutrition? ASPEN website. Accessed September 5, 2024. https://www.nutritioncare.org/About_Clinical_Nutrition/What_Is_Parenteral_Nutrition_/ 4. Data on File; 11/1/24; calculation includes: all ILEs approved in the US. 5. SMOFlipid Prescribing Information, Fresenius Kabi USA, LLC. 2023. 6. Kalish BT, Fallon EM, Puder M. A tutorial on fatty acid biology. JPEN J Parenter Enteral Nutr. 2012;36(4):380-388. 7. Deckelbaum RJ, Hamilton JA, Moser A, et al. Medium-chain versus long-chain triacylglycerol emulsion hydrolysis by lipoprotein lipase and hepatic lipase: implications for the mechanisms of lipase action. Biochemistry. 1990;29(5):1136-1142. 8. Omegaven Prescribing Information, Fresenius Kabi USA, LLC. 2023.

The nurturing role of neonatal nurses
More than 3 million babies are born each year in the US.1 While many are born healthy, some come into the world with a range of issues, including birth defects, prematurity, infections, and heart or surgical problems.2 These tiny patients require careful nurturing, which is readily provided by the heroes of the neonatal intensive care unit (NICU): neonatal nurses.
Making a difference in new lives
Neonatal nurses care for babies shortly after birth; however, comprehensive care extends to these little ones who are sick for months or have long-term issues associated with prematurity or an illness.2,3 Some neonatal nurses may even care for toddlers up to about two years of age, but most care for babies from the time of birth until they can be safely discharged from the hospital.2,3
Neonatal nurses deliver around-the-clock care to critically ill newborns in a number of ways, including3:

Bathing, feeding, and diapering
Administering medications and therapies
Monitoring vital signs
Adjusting the settings on medical equipment

Identifying and notifying the neonatologist of any signs of distress

Providing education and guidance to parents on caring for their babies
Coordinating care plans

Documenting baby’s condition and interventions
“Every parent and patient are different. It’s important to assess what their educational needs are and what the needs are for each baby. First-time parents may need more education around newborn care, feeding, safe sleep, developmental milestones, etc. compared to those who have experience.”
-Taryn M. Edwards, MSN, APRN, NNP-BC
Going beyond the clinical skills
Seeing their baby hooked up to wires, making difficult medical decisions, and not knowing what the future holds are some of the many challenges that parents are faced with in the NICU.3 While neonatal nurses possess the clinical skills needed to care for sick newborns, they also provide emotional support to parents to help them navigate this stressful period in their lives.3
They often lend their support by3:

Engaging in kangaroo care (skin-to-skin contact) to facilitate parent-baby bonding

Educating parents on how they can become involved in their baby’s care

Celebrating milestones
Encouraging hope during trying times

Empathetically listening to parents’ fears and concerns
Connecting families with resources and support groups
Advocating for the needs of the families
Neonatal nurses and parenteral nutrition (PN)
When it comes to pediatric clinical nutrition, neonatal nurses are actively involved. Some newborns have medical conditions that warrant the need for PN to help them grow. Because PN is a complex process,4 neonatal PN services are provided by a specialist multidisciplinary team that consists of a neonatologist or pediatrician, a neonatal dietitian, and a neonatal pharmacist.5 A neonatal nurse is also readily available to support the team in nourishing babies with PN, monitoring clinical outcomes, and providing input for babies with complex needs.5
“Parenteral nutrition is a staple in the NICU and is needed for those infants that are unable to receive full enteral nutrition. For most of our vulnerable infants in the NICU, it is lifesaving.”
-Taryn M. Edwards, MSN, APRN, NNP-BC
Thank you, neonatal nurses
While neonatal nurses are officially recognized and celebrated in September, we appreciate all that they do to nurture brand new lives every day. Their tremendous compassion and unwavering dedication are simply unmatched. Fresenius Kabi thanks each one of them for caring for the tiniest patients.
Sources: 1. How many babies are born each year in the US? Unicef website. Accessed May 30, 2024. https://data.unicef.org/how-many/how-many-babies-are-born-each-year-in-the-us/ 2. What is Neonatal Nursing? NANN website. Accessed May 30, 2024. https://nann.org/about/what-is-neonatal-nursing#:~:text=Neonatal%20nursing%20is%20a%20subspecialty,are%20often%20sick%20for%20months 3. Exploring the Vital Role of NICU Nurses in Neonatal Care. Health Carousel Nursing & Allied Health website. Accessed May 31, 2024. https://www.hctravelnursing.com/blog/nicu-nurse-duties#:~:text=NICU%20nurses%20monitor%20vital%20signs,to%20promote%20growth%20and%20bonding 4. Boullata JI. Overview of the parenteral nutrition use process. JPEN J Parenter Enteral Nutr. 2012;36(2 Suppl):10S-13S. 5. Neonatal parenteral nutrition. London: National Institute for Health and Care Excellence (NICE); 2020 Feb 26. (NICE Guideline, No. 154.) Available from: https://www.ncbi.nlm.nih.gov/books/NBK555677/

Examining nutritional guidance in the pediatric intensive care unit (PICU)
Malnutrition is common in hospitalized children, including those admitted to the PICU.1,2 According to one meta-analysis of 15 studies involving 4,331 study participants, the pooled prevalence of malnutrition among pediatric critically ill patients was more than 37%.3 That’s an alarming number, and the adverse outcomes may be severe. Inadequate nutrition can result in “loss of lean body mass, muscle weakness, developmental/intellectual delays, infections, immune dysfunction, delayed wound healing, prolonged length of hospital stay, and even death.”1
The causes of pediatric malnutrition are multifactorial—a disease, an injury, or even socioeconomics can hinder a child’s ability to receive adequate nutrition.1,4 Whatever the reason may be, appropriate nutrition support therapy in the PICU, including parenteral nutrition (PN), is an important consideration for helping these young patients grow. Learn more about the role of PN in pediatric malnutrition in our blog.
Because malnutrition is prevalent in critically ill children, and high-level evidence for nutrition practices in the PICU have been scarce, the Society of Critical Care Medicine (SCCM) and the American Society for Parenteral and Enteral Nutrition (ASPEN) developed guidelines in 2017 that offer recommendations for nutrition support in these vulnerable patients.2 They evaluated literature, expert opinions, and clinical practices to address some of the questions regarding best practices for nutrition screening, use of PN, and more.2
Let’s dive into some of their recommendations.

What is the impact of nutrition status on outcomes?
“Based on observational studies, malnutrition, including obesity, is associated with adverse clinical outcomes, including longer periods of ventilation, higher risk of hospital-acquired infection, longer PICU and hospital stay, and increased mortality. We recommend that patients in the PICU undergo detailed nutrition assessment within 48 hours of admission.
Furthermore, as patients are at risk of nutrition deterioration during hospitalization, which can adversely affect clinical outcomes, we suggest that the nutrition status of patients be reevaluated at least weekly throughout hospitalization.
Quality of evidence. Very low.
GRADE recommendation. Strong.”2
What are the best practices to screen and identify patients with malnutrition or those at risk of nutrition deterioration?
“On the basis of observational studies and expert consensus, we recommend that weight and height/length be measured at admission to the PICU and that z scores for body mass index (BMI) for age (weight for length, <2 years) or weight for age (if accurate height is not available) be used to screen for patients at extremes of these values. For children <36 months old, head circumference must be documented.
Validated screening methods for the PICU population to identify patients at risk of malnutrition must be developed. Screening methods might allow limited resources to be directed to high-risk patients who are most likely to benefit from early nutrition interventions.
Quality of evidence. Very low.
GRADE recommendation. Strong.”2
Fresenius Kabi: at the forefront of PN
As pioneers in clinical nutrition, we have innovations that nourish patients of all ages who require PN. Learn more about our dedication to providing more options for more patients: www.FreseniusKabiNutrition.com
Sources: 1. Goldberg DL, Van Poots HA. Pediatric and Neonatal Malnutrition: A Collaborative, Family-Centered Approach Improves Outcomes. Pediatr Neonatal Nurs Open J. 2019;6(1):e1-e4. 2. Mehta NM, Skillman HE, Irving SY, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Pediatric Critically Ill Patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr. 2017;41(5):706-742. 3. Abera EG, Sime H. The prevalence of malnutrition among critically ill children: a systematic review and meta-analysis. BMC Pediatr. 2023;23(1):583. Published 2023 Nov 21. 4. Mehta NM, Corkins MR, Lyman B, et al. Defining pediatric malnutrition: a paradigm shift toward etiology-related definitions. JPEN J Parenter Enteral Nutr. 2013;37(4):460-481.

Exploring advancements in parenteral nutrition (PN)
PN is used to help nourish critically and chronically ill patients all around the world. This lifesaving therapy has evolved over the years, with several innovative options now available on the market that help nourish more than 33,000 US patients in the hospital and approximately 25,000 patients at home.1,2
A tireless dedication to driving advancements has led to many firsts in the field. From developing the first and only 3-chamber bag for adult PN to pioneering the use of fish oil and omega-3s in PN products to introducing the only pediatric lipid injectable emulsion (ILE) for parenteral nutrition-associated cholestasis (PNAC), Fresenius Kabi is committed to bringing innovation and advancements to clinicians and patients. Let’s take a closer look at our comprehensive portfolio of innovative clinical nutrition solutions.
Our PN products are used to help care for critically and chronically ill patients in hospitals, long-term care facilities, and at home.

With SMOFlipid, Fresenius Kabi supported PN and critical care medical societies’ need for an alternative to soybean-sparing ILEs. This blend of soybean oil, medium-chain triglycerides, olive oil, and fish oil is the FIRST and ONLY 4-oil ILE for infants, children, teenagers, and adults.3 SMOFlipid has a well-established safety and tolerability profile.3 In addition, this globally recognized innovation has been administered to more than 7 million patients worldwide* for over 15 years.
See what else sets SMOFlipid apart:
For Adults: www.FreseniusKabiNutrition.com/products/smoflipid-adults/
For Pediatrics: www.FreseniusKabiNutrition.com/products/smoflipid-pediatrics/

Omegaven is the FIRST and ONLY PN emulsion for pediatric patients with PNAC.4 It is the only innovation that nourishes with 100% fish oil,4 which is the newest type of lipid to be introduced into PN products. Previously available only for compassionate care, we worked hard to secure the necessary clinical evidence to support FDA approval of Omegaven, demonstrating our unwavering commitment to supporting advancements in PN.
Discover how Omegaven is making a splash in the sea of clinical nutrition: www.FreseniusKabiNutrition.com/products/Omegaven/
A long-standing and chosen lipid worldwide, Intralipid nourishes children and adults in the hospital with 100% soybean oil.5 It has been administered in more than 200 million infusions since its European approval in 1962.6 Intralipid may be considered for patients requiring PN as a source of essential fatty acids, for prevention of essential fatty acid deficiency, and when other lipid sources are not an option.5
Learn more: www.FreseniusKabiNutrition.com/products/Intralipid/

As the FIRST and ONLY 3-chamber bag for adult PN,7,8 Kabiven/Perikabiven’s unique design streamlines the delivery of nutrition therapy to patients by simplifying calculations, prescription writing, compounding, and administration, all while supporting PN safety by minimizing the risk of contamination.9 This all-in-one solution helps clinicians efficiently deliver 3 macronutrients—dextrose, protein, and lipids—plus electrolytes in volumes and concentrations that meet the needs of most adult PN patients.7,8
Explore the convenience of the three-chamber bag: www.FreseniusKabiNutrition.com/products/kabiven-perikabiven/
Fresenius Kabi is dedicated to putting innovative PN solutions in the hands of those who care for patients. From infants to toddlers to teenagers to adults, our innovations nourish critically and chronically ill patients—from hospital to home.
SMOFlipid® (lipid injectable emulsion, USP), for intravenous use
IMPORTANT SAFETY INFORMATION FOR CONSUMERS
What is SMOFlipid?
- Indicated in adult and pediatric patients as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
- The hourly infusion rate in pediatrics should not exceed 0.75 mL/kg/hour and 0.5 mL/kg/hour in adults.
SMOFlipid should not be received by patients who have:
- A known allergy to fish, egg, soybean, or peanut, or to any of the active or inactive ingredients in SMOFlipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
SMOFlipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (>1%) in adult patients include nausea, vomiting, and high levels of glucose in the blood and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase) and hospital-acquired infections.
These are not all the possible side effects associated with SMOFlipid. Call your healthcare provider for medical advice regarding SMOFlipid side effects. You are encouraged to report negative side effects of SMOFlipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at https://freseniuskabinutrition.com/SMOFlipidPI.
OMEGAVEN (fish oil triglycerides) injectable emulsion, for intravenous use
IMPORTANT SAFETY INFORMATION FOR CONSUMERS
These highlights do not include all the information needed to use OMEGAVEN safely and effectively. To learn more about OMEGAVEN for your child, talk to your child’s healthcare provider. OMEGAVEN is available by prescription only. The FDA-approved product labeling can be found at www.freseniuskabinutrition.com/OmegavenPI.
What is OMEGAVEN?
- A fish oil-based intravenous lipid emulsion that is a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
- Does not prevent PNAC.
- It has not been demonstrated that the clinical outcomes seen in pediatric patients are a result of the omega-6:omega-3 fatty acid ratio of the product.
- The hourly infusion rate should not exceed 1.5 mL/kg/hour
OMEGAVEN should not be received by patients who have:
- a known allergy to fish or egg protein or to any of the ingredients in OMEGAVEN.
- a severe bleeding disorder.
- abnormally high levels of lipid (triglycerides) in the blood.
What important safety information should I know about OMEGAVEN?
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
The most common side effects, (>15%) include: vomiting, agitation, slower than normal heartbeat, interruption of breathing, and viral infection.
These are not all the possible side effects associated with OMEGAVEN. Call your healthcare provider for medical advice regarding OMEGAVEN side effects. You are encouraged to report negative side effects of OMEGAVEN. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.FreseniusKabiNutrition.com/OmegavenPI.
Intralipid (lipid injectable emulsion) for intravenous use
IMPORTANT SAFETY INFORMATION FOR CONSUMERS
What is Intralipid?
- Indicated as a source of calories and essential fatty acids for adult and pediatric patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
Intralipid should not be received by patients who have:
- A known allergy to egg, soybean, or peanut, or any of the active ingredients or excipients in Intralipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
Intralipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly adhere to the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides: Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (≥5%) in adult patients include nausea, vomiting and fever and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase), and cholestasis (i.e., reducing or blocking the flow of bile).
These are not all the possible side effects associated with Intralipid. Call your healthcare provider for medical advice regarding Intralipid side effects. You are encouraged to report negative side effects of Intralipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.FreseniusKabiNutrition.com/IntralipidPI.
KABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
PERIKABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
IMPORTANT SAFETY INFORMATION FOR CONSUMERS
What is Kabiven and Perikabiven?
- Indicated in adult patients as a source of calories, protein, electrolytes and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adults.
- Do not exceed the recommended maximum infusion rate of 2.6 mL/kg/hour for Kabiven and 3.7 mL/kg/hour for Perikabiven.
Limitations of Use
Neither Kabiven nor Perikabiven is recommended in pediatric patients less than 2 years old because the fixed amount of the formulations do not meet nutritional needs in this age group.
Do not use Kabiven or Perikabiven in patients who have:
- Simultaneous treatment with ceftriaxone in neonates (28 days of age or younger)
- Known allergy to egg, soybean, peanut or any of the active or inactive ingredients
- Abnormally high levels of lipid (triglycerides) in the blood (with serum triglyceride concentration >1,000 g/dL)
- Inborn errors of amino acid metabolism (a genetic defect in protein metabolism)
- Cardiopulmonary instability (inability for the heart and lungs to function right)
- Hemophagocytic syndrome (a disorder of the immune system)
Kabiven and Perikabiven may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who receive parenteral nutrition for greater than 2 weeks. Your healthcare provider will monitor liver tests.
- Pulmonary Embolism (a blockage in a blood vessel in the lung) and Respiratory Distress (increased breathing rate, bluish skin color changes, wheezing) due to Pulmonary Vascular Precipitates (solid substance in the blood vessel of the lungs): If signs of lung issues occur, stop the infusion and start a medical evaluation.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction
- Precipitation (solid substance in the blood vessel) with Ceftriaxone: Do not administer ceftriaxone simultaneously with Kabiven or Perikabiven via a Y-site.
- Infection, fat overload, hyperglycemia (high blood sugar) and refeeding syndrome: Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels
The most common adverse reactions for Kabiven (≥3%) are nausea, fever, high blood pressure, vomiting, decreased blood hemoglobin, decreased blood total protein, low blood potassium, and increased gamma glutamyltransferase (a liver enzyme). The most common adverse reactions for Perikabiven (≥3%) are high blood sugar, low blood potassium, fever and increased blood lipids.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Tell your doctor if you are taking coumarin and coumarin derivatives, including warfarin: the drug activity may be lessened and your healthcare provider will monitor your blood.
These are not all the possible side effects associated with Kabiven and Perikabiven. Call your healthcare provider for medical advice regarding Kabiven and Perikabiven side effects. You are encouraged to report negative side effects of Kabiven and Perikabiven. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at www.FreseniusKabiNutrition.com/KabivenPI and www.FreseniusKabiNutrition.com/PerikabivenPI.
*Data on file 3/1/24.
Sources: 1. John J, Seifi A. Total parenteral nutrition usage trends in the United States. J Crit Care. 2017;40:312-313. 2. Mundi MS, Pattinson A, McMahon MT, Davidson J, Hurt RT. Prevalence of Home Parenteral and Enteral Nutrition in the United States. Nutr Clin Pract. 2017;32(6):799-805. 3. SMOFlipid Prescribing Information, Fresenius Kabi USA, LLC. 2023. 4. Omegaven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 5. Intralipid Prescribing Information, Fresenius Kabi USA, LLC. 2023. 6. Data on file. 7. Kabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 8. Perikabiven Prescribing Information, Fresenius Kabi USA, LLC. 2023. 9. Derenski K. Standardize parenteral nutrition using commercial premix. Pharm Purchasing Prod. 2012;1-7.