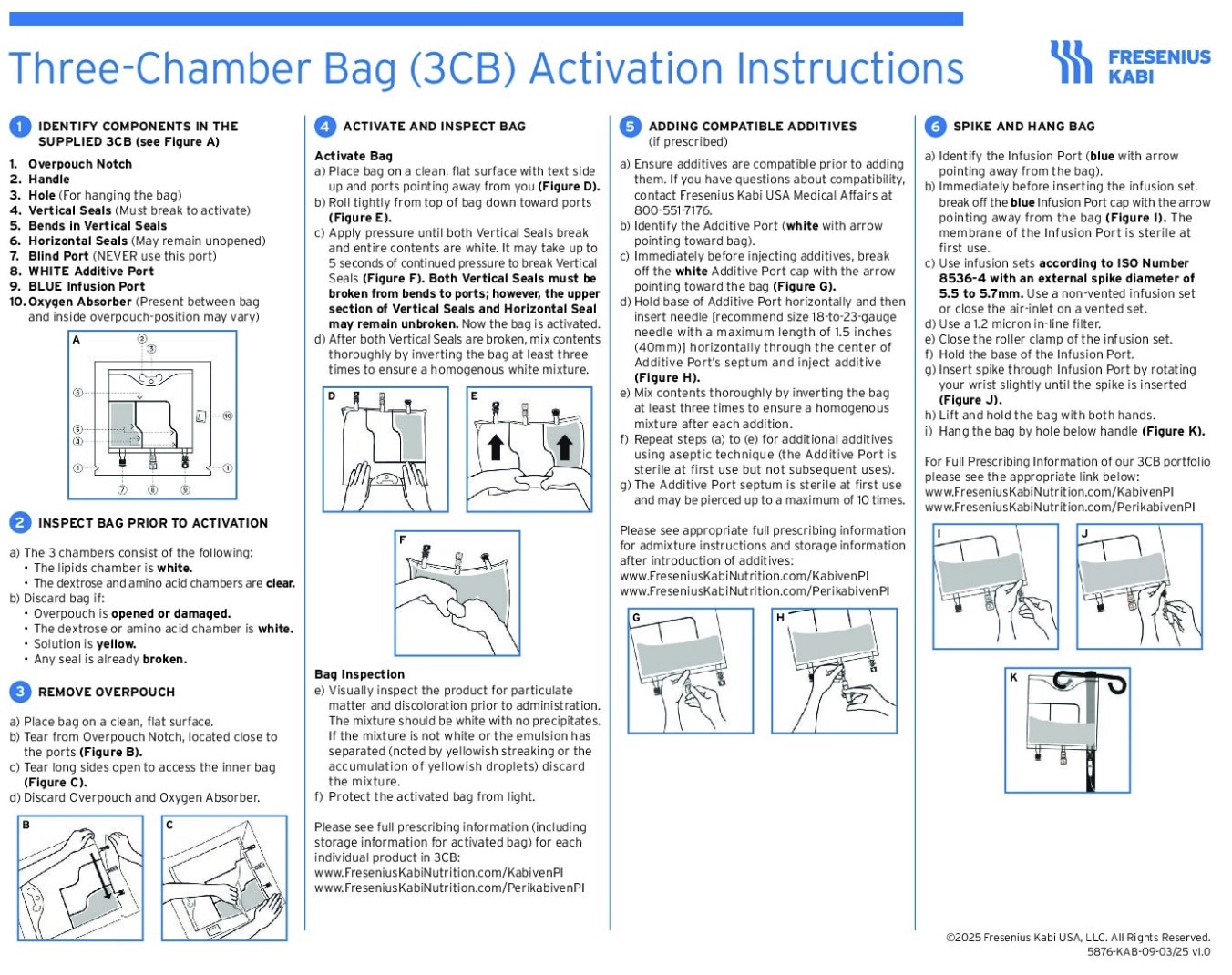
Explore innovative 3-chamber bags (3CBs) for adult parenteral nutrition (PN)1,2
Kabiven: Full prescribing information>
Perikabiven: Full prescribing information>
Download the composition chart
Features of Kabiven/Perikabiven
The Kabiven and Perikabiven 3-chamber PN bags help clinicians efficiently deliver 3 macronutrients (dextrose, amino acids, and lipids) plus electrolytes in volumes and concentrations that may meet the needs of adult PN patients by reducing waste.1-3
- Two formulations for added flexibility:
- Kabiven for central PN1
- Perikabiven for peripheral or central PN2
- Eliminates the need to piggyback lipids
- Be dispensed by the pharmacy anytime, including nights and weekends
- Provide a full complement of electrolytes
- Require less manipulation during preparation and administration4
- No need to hang lipids separately
A time and motion study evaluated time, labor, and cost savings of 3CBs compared with hospital-compounded bags (HCBs)3
In a multicenter, prospective, time and motion study evaluating PN delivery systems, the 3CB delivery system was associated with a 62% reduction in pharmacy staff time and workload as well as a 37% reduction in costs compared with HCBs (representing PN prepared with automated compounding devices). The cost per bag included labor, PN products, medical consumables, and equipment. One hundred thirty-six PN prescriptions were prepared during the study (66 for 3CBs and 70 for HCBs).3
An all-in-one solution in a 3CB bag may help simplify:
Calculations for dietitians
Compounding for pharmacists
Prescription writing for clinicians
Administration for nurses
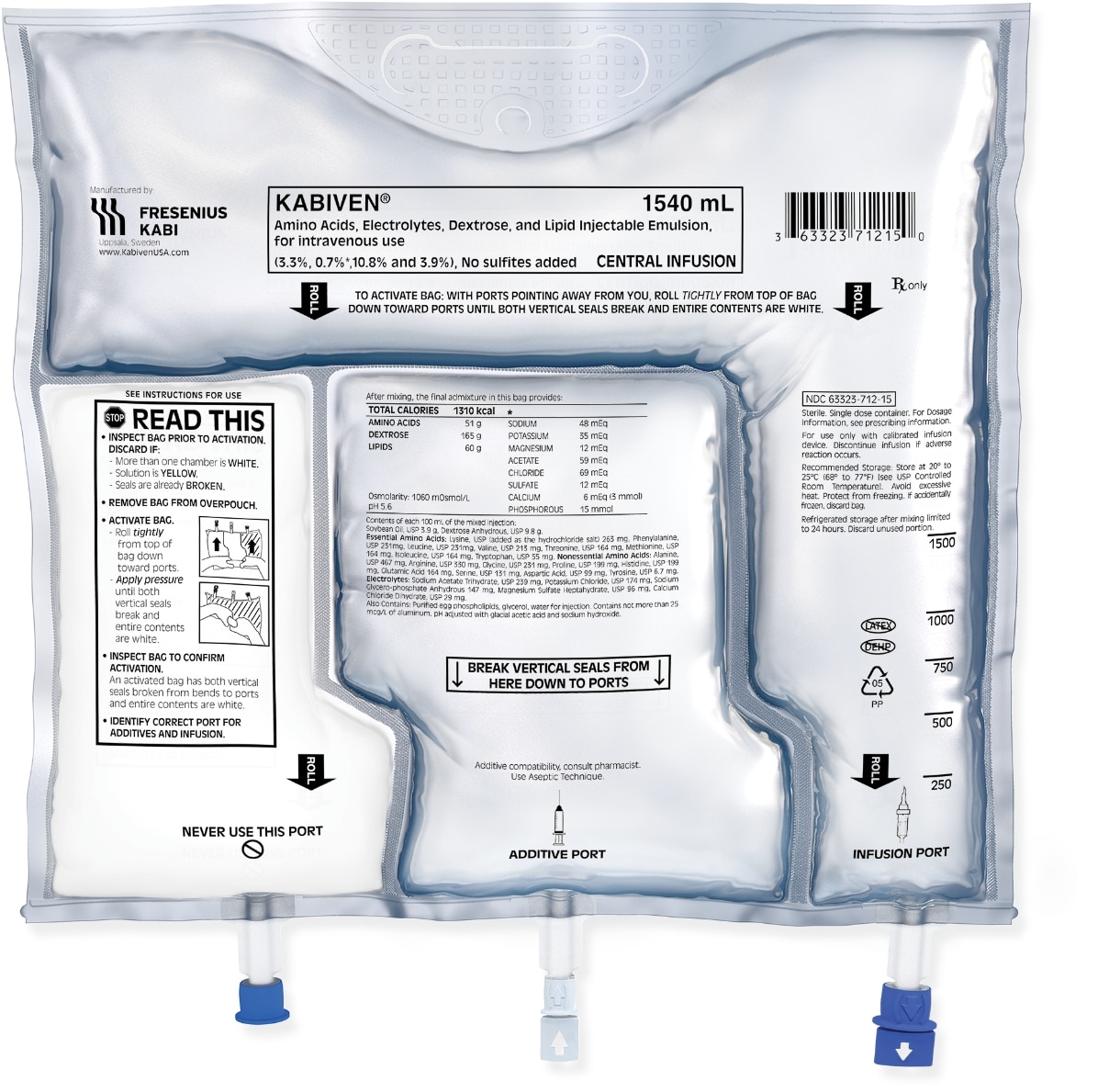
3-chamber bag setup
3-chamber bag ports are color coded and marked with arrows for infusion/additive port. The infusion port utilizes flow-stop, which helps prevent leakage after removal of the needle or spike
- 1 PN bag
- 1 DEHP-free IV tubing set with an integrated or added 1.2 micron filter
- 1 infusion pump
- 1 rate on the pump
2-chamber bag with Y-site lipid setup
2-chamber bag ports are not marked, and the infusion ports are not resealable
- 2 PN bags
(amino acids/dextrose/electrolytes + lipids) - 2 non-vented DEHP-free IV tubing sets
(1 with a 1.2 micron filter) - 2 infusion pumps
- 2 rates on the pumps
Kabiven/Perikabiven resources
Explore additional Kabiven/Perikabiven materials in our Resource Center.
Designed for convenience

Fresenius Kabi pioneered the use of an all-in-one 3CB designed for the efficient delivery of PN.
- Innovative design keeps components separated until bag is ready for activation
- Up to 24-month shelf life
- Clear overwrap allows for quick visual inspection
- Additive and infusion ports are sterile for the first use
- Self-sealing additive and infusion ports have a semi-rigid port design
Indications and limitations of use
Indication:
Kabiven and Perikabiven are each indicated as a source of calories, protein, electrolytes, and essential fatty acids for adult patients requiring PN when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adult patients.1,2
Kabiven is indicated for intravenous infusion into a central vein.1 Perikabiven is indicated for intravenous infusion into a peripheral or central vein.2
Limitations of Use:
Neither Kabiven nor Perikabiven are recommended for use in pediatric patients <2 years, including preterm infants because the fixed content of the formulations do not meet the nutritional requirements in this age group.1,2
For Consumers
KABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
PERIKABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
IMPORTANT SAFETY INFORMATION
What is Kabiven and Perikabiven?
- Indicated in adult patients as a source of calories, protein, electrolytes and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adults.
- Do not exceed the recommended maximum infusion rate of 2.6 mL/kg/hour for Kabiven and 3.7 mL/kg/hour for Perikabiven.
Limitations of Use
Neither Kabiven nor Perikabiven is recommended in pediatric patients less than 2 years old because the fixed amount of the formulations do not meet nutritional needs in this age group.
Do not use Kabiven or Perikabiven in patients who have:
- Simultaneous treatment with ceftriaxone in neonates (28 days of age or younger)
- Known allergy to egg, soybean, peanut or any of the active or inactive ingredients
- Abnormally high levels of lipid (triglycerides) in the blood (with serum triglyceride concentration >1,000 g/dL)
- Inborn errors of amino acid metabolism (a genetic defect in protein metabolism)
- Cardiopulmonary instability (inability for the heart and lungs to function right)
- Hemophagocytic syndrome (a disorder of the immune system)
For Consumers
KABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
PERIKABIVEN (Amino Acids, Electrolytes, Dextrose, and Lipid Injectable Emulsion), for intravenous use
IMPORTANT SAFETY INFORMATION
What is Kabiven and Perikabiven?
- Indicated in adult patients as a source of calories, protein, electrolytes and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated. Kabiven and Perikabiven may be used to prevent essential fatty acid deficiency or treat negative nitrogen balance in adults.
- Do not exceed the recommended maximum infusion rate of 2.6 mL/kg/hour for Kabiven and 3.7 mL/kg/hour for Perikabiven.
Limitations of Use
Neither Kabiven nor Perikabiven is recommended in pediatric patients less than 2 years old because the fixed amount of the formulations do not meet nutritional needs in this age group.
Do not use Kabiven or Perikabiven in patients who have:
- Simultaneous treatment with ceftriaxone in neonates (28 days of age or younger)
- Known allergy to egg, soybean, peanut or any of the active or inactive ingredients
- Abnormally high levels of lipid (triglycerides) in the blood (with serum triglyceride concentration >1,000 g/dL)
- Inborn errors of amino acid metabolism (a genetic defect in protein metabolism)
- Cardiopulmonary instability (inability for the heart and lungs to function right)
- Hemophagocytic syndrome (a disorder of the immune system)