PNAC in preterm infants: balancing nutrition, obstacles, and resources
Preterm infants often rely on parenteral nutrition (PN) to grow while the gut matures.1 In some cases, complications such as necrotizing enterocolitis (NEC) or congenital anomalies can lead to intestinal failure, making long-term PN essential.2
PN is life-sustaining but can also be expensive.3 A cross-sectional study of children with neonatal short bowel syndrome and intestinal failure (2004–2020) reported median initial hospitalizations of 150 days, with median costs exceeding $500,000.4
For many of these children, PN dependence continues well beyond the neonatal period, and growth remains a persistent challenge—about half of children with intestinal failure experience growth failure.5,6 A prolonged dependence on PN also increases the risk of complications such as parenteral nutrition–associated cholestasis (PNAC), often defined by a direct bilirubin ≥2 mg/dL.7
In severe cases, PNAC can progress to liver failure requiring transplantation and total medical costs exceeding more than $600,000 for commercially and Medicaid-insured patients within the first 3 months after surgery.8,9
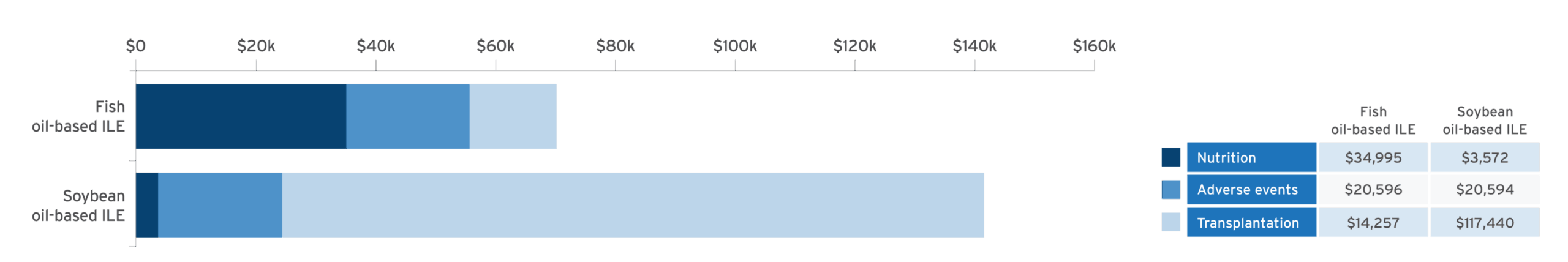
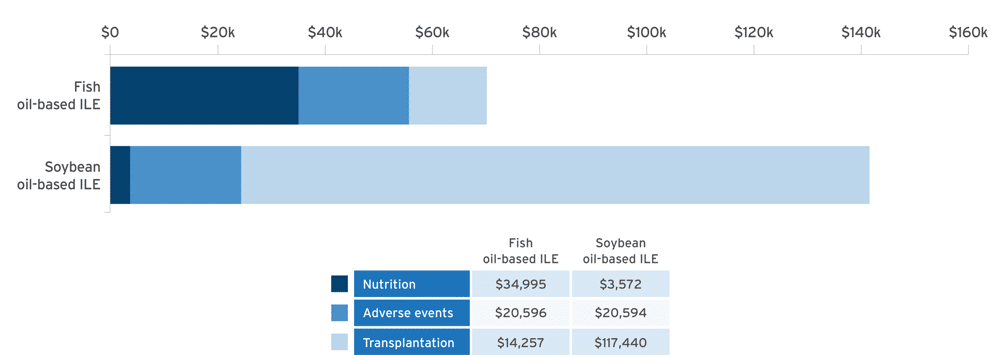
Economic modeling of estimated cost savings of fish oil lipid emulsion (FOLE) vs soybean oil lipid emulsion (SOLE) in PNAC10:
Adapted from Povero M, et al. JPEN J Parenter Enteral Nutr. 2025;49(2):180-188.10
A discrete event simulation model evaluated cost‐effectiveness by simulating clinical outcomes in 10,000 patients and estimating associated healthcare costs in pediatric patients with PNAC receiving PN with FOLE (1 g/kg/day) or SOLE (1.9 g/kg/day) over a time horizon of 6 years.10
- Results confirmed with probabilistic sensitivity analysis (95% confidence interval [CI])10
- Cost of Omegaven® (fish oil triglycerides) injectable emulsion, for intravenous use, was offset by the reduction in costs by avoidance of liver transplant10
Study limitations10
- Data were estimated using a historical cohort that received Intralipid® 20% (lipid injectable emulsion), for intravenous use, resulting in a difference of treatment eras
- Only the mortality rate was reported in the combined database, with no overall survival curves available
- Lack of longitudinal data for children with PNAC and precise data to estimate the cost of treatment for specific adverse events
Risk of Infections: Monitor for signs and symptoms; monitor laboratory parameters.11
Most common adverse drug reactions (>15%) are: vomiting, agitation, bradycardia, apnea and viral infection.11
Why this matters to healthcare systems and families
PN can be demanding—for patients, families, and the health system. Even small shifts in therapy can create ripple effects. While care must always be individualized, economic modeling studies offer perspective on how nutritional strategies may influence both financial outcomes and resources.
Could a fish oil lipid emulsion be right for your patients with PNAC?
About Omegaven® (fish oil triglycerides) injectable emulsion, for intravenous use
Omegaven is the only 100% fish oil lipid emulsion approved in the US for pediatric patients with PNAC. It provides omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).11 Omegaven may be used as part of a balanced PN regimen tailored to individual patient needs.
Please see the full Prescribing Information for Omegaven.
OMEGAVEN (fish oil triglycerides) injectable emulsion, for intravenous use
IMPORTANT SAFETY INFORMATION
These highlights do not include all the information needed to use OMEGAVEN safely and effectively. To learn more about OMEGAVEN for your child, talk to your child’s healthcare provider. OMEGAVEN is available by prescription only. The FDA-approved product labeling can be found at https://freseniuskabinutrition.com/OmegavenPI.
What is OMEGAVEN?
- A fish oil-based intravenous lipid emulsion that is a source of calories and fatty acids in pediatric patients with parenteral nutrition-associated cholestasis (PNAC).
- Does not prevent PNAC.
- It has not been demonstrated that the clinical outcomes seen in pediatric patients are a result of the omega-6:omega-3 fatty acid ratio of the product.
- The hourly infusion rate should not exceed 1.5 mL/kg/hour
OMEGAVEN should not be received by patients who have:
- a known allergy to fish or egg protein or to any of the ingredients in OMEGAVEN.
- a severe bleeding disorder.
- abnormally high levels of lipid (triglycerides) in the blood.
What important safety information should I know about OMEGAVEN?
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly follow the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides (Hypertriglyceridemia): Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels.
- The most common side effects, (>15%) include: vomiting, agitation, slower than normal heartbeat, interruption of breathing, and viral infection.
- These are not all the possible side effects associated with OMEGAVEN. Call your healthcare provider for medical advice regarding OMEGAVEN side effects. You are encouraged to report negative side effects of OMEGAVEN. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling can be found at https://freseniuskabinutrition.com/OmegavenPI.
Intralipid (lipid injectable emulsion) for intravenous use
IMPORTANT SAFETY INFORMATION
What is Intralipid?
- Indicated as a source of calories and essential fatty acids for adult and pediatric patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
Intralipid should not be received by patients who have:
- A known allergy to egg, soybean, or peanut, or any of the active ingredients or excipients in Intralipid.
- Abnormally high levels of lipid (triglycerides) in the blood.
Intralipid may cause serious side effects including:
- Serious Adverse Reactions with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Strictly adhere to the recommended total daily dosage and do not exceed the maximum infusion rate. If poor clearance of fats occurs, the infusion should be stopped, and a medical evaluation started.
- Risk of Parenteral Nutrition-Associated Liver Disease: Parenteral nutrition-associated liver disease (PNALD) may progress to liver inflammation and damage caused by a buildup of fat in the liver with scarring and cirrhosis.
- Allergic Reactions: Contact your healthcare provider immediately if you are experiencing an allergic reaction.
- Fat Overload Syndrome, Refeeding Syndrome, Elevated Triglycerides: Your healthcare provider will monitor you for signs and symptoms of early infection and blood levels
Monitoring/Laboratory Tests: The content of vitamin K may interfere with blood clotting activity of medications.
The most common side effects (≥5%) in adult patients include nausea, vomiting and fever and in pediatric patients include low levels of red blood cells, vomiting, increased levels of liver enzymes (i.e., gamma-glutamyltransferase), and cholestasis (i.e., reducing or blocking the flow of bile).
These are not all the possible side effects associated with Intralipid. Call your healthcare provider for medical advice regarding Intralipid side effects. You are encouraged to report negative side effects of Intralipid. Contact Fresenius Kabi USA, LLC at: 1-800-551-7176 or FDA at: 1-800-FDA-1088 or www.fda.gov/medwatch. The FDA-approved product labeling for Intralipid® 20% (lipid injectable emulsion), for intravenous use and Intralipid® 30% (lipid injectable emulsion), for intravenous use can be found at www.FreseniusKabiNutrition.com/Intralipid20PI and www.FreseniusKabiNutrition.com/Intralipid30PI.