

What a recent study reveals about intravenous lipid use in high-risk neonates requiring parenteral nutrition (and associated hepatic outcomes)
A recent randomized, controlled trial published in The Journal of Nutrition sheds light on how lipid injectable emulsion (ILE) choice may affect liver health, fatty acid status, and growth.
The study at a glance
A multicenter, randomized, double-blind, controlled trial enrolled 161 hospitalized neonates and infants across 14 sites in the US. All participants were expected to require at least 28 days of parenteral nutrition (PN) due to gastrointestinal conditions or surgical complications. The study compared a composite, fish oil-containing lipid emulsion (n=83) to a traditional soybean oil-based emulsion (n=78) as part of a full PN regimen.
-

Key details
Population: Term and preterm neonates and infants (≥24 weeks gestational age, ≥750 g birth weight) requiring ≥28 days of PN.
Intervention: The targeted lipid dose for PN was 3.0 g/kg body weight/d administered over 20 to 24 hours by central or peripheral vein infusion (mean lipid dose 2.0 ± 0.1 g/kg/d composite ILE, 2.6 ± 0.2 g/kg/d traditional soybean oil-based ILE).
Primary endpoint: Incidence of cholestasis, defined as conjugated bilirubin >2 mg/dL confirmed by a second sample 7 days later.
Secondary endpoints: Length of stay in the hospital and conjugated bilirubin concentrations in plasma.
-
Liver results
While the overall incidence of cholestasis was low across both groups—reflecting improvements in neonatal care—a few trends emerged:
- Patients who received the composite ILE had significantly lower conjugated bilirubin levels at the end of the initial treatment phase (P=0.006).
- No new cases of confirmed cholestasis were observed after day 28 in the composite ILE group.
- Patients receiving the composite ILE showed a trend toward a decreased risk of intestinal failure-associated liver disease (IFALD).
-

Fatty acid profiles
The study also measured changes in plasma and red blood cell polyunsaturated fatty acids (PUFAs). Findings include:
- Serum eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) levels increased at the end of the initial and extended treatment phases in patients receiving the composite ILE.
- No cases of essential fatty acid deficiency (EFAD) in either group until the end of the 84-day extended treatment phase based on the Holman index.*
*Insufficient data to determine the incidence of EFAD with SMOFlipid® >28 days. Cases of EFAD have been reported in adult and pediatric patients in the postmarketing period with SMOFlipid.
-

Clinical outcomes: growth, feeding, and length of stay
Despite more small-for-gestational-age infants in the composite group at baseline, both groups showed similar growth trajectories. Additional findings included:
- Comparable rates of transition to full enteral feeds.
- Overall median time to discharge from the hospital was 56.7 days for the composite ILE group and 66.4 days for the traditional soybean oil-based ILE group (secondary outcome, NS).
Why this matters
The data suggest that a composite ILE could be considered an alternative to a traditional soybean oil-based ILE in high-risk neonates.
Study limitations
There was a low incidence of cholestasis in both treatment groups compared to historical data. Additionally, there was a low completion rate (40%) predominantly due to earlier weaning from PN. The use of SMOFlipid in patients with established cholestasis was not assessed.
Read the full article here.
Abrams SA, Ernst KD, Weitkamp JH, et al. Safety and Efficacy of a Composite Lipid Emulsion with Fish Oil in Hospitalized Neonates and Infants Requiring Prolonged Parenteral Nutrition – A Randomized, Double-Blind, Multicenter, Controlled Trial. J Nutr. 2024;154(12):3615-3625.
This trial was funded by Fresenius Kabi Deutschland GmbH upon requirement of a postmarketing study from the United States Food and Drug Administration.
INDICATIONS AND USAGE
SMOFlipid is indicated in adult and pediatric patients, including term and preterm neonates, as a source of calories and essential fatty acids for parenteral nutrition (PN) when oral or enteral nutrition is not possible, insufficient, or contraindicated.
IMPORTANT SAFETY INFORMATION
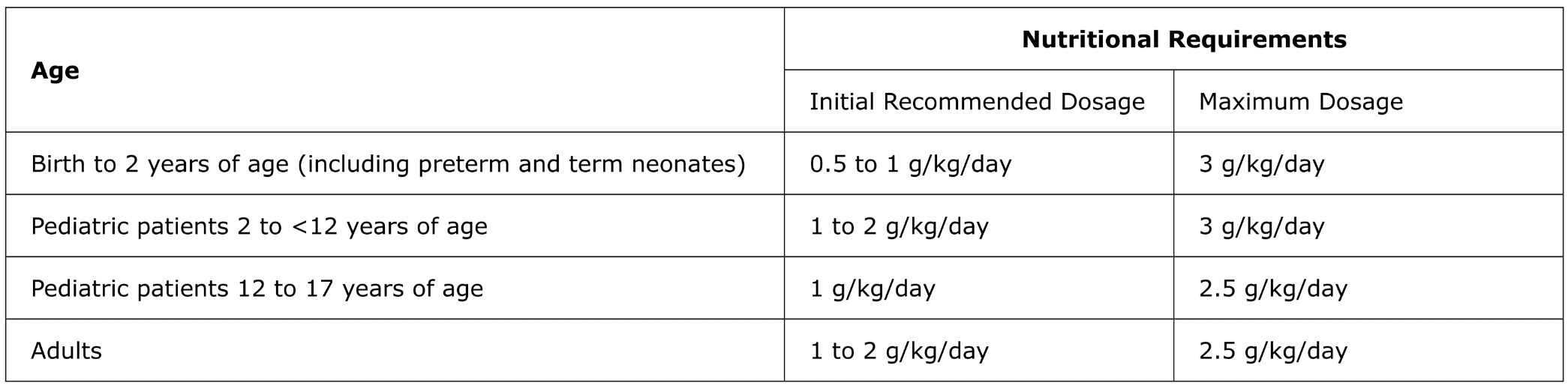
For intravenous infusion only into a central or peripheral vein. Use a non-vented non-DEHP 1.2 micron in-line filter set during administration. Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient. The recommended dose for adults and pediatrics is shown in Table 1. For information on age-appropriate infusion rate, see the full prescribing information. SMOFlipid Pharmacy Bulk Package is only indicated for use in pharmacy admixture programs for the preparation of three-in-one or total nutrition admixtures. Protect the admixed PN solution from light.
Table 1: Recommended Adult and Pediatric Dosage
SMOFlipid is contraindicated in patients with known hypersensitivity to fish, egg, soybean, peanut, or any of the active or inactive ingredients, and severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglycerides >1,000 mg/dL).
Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported.
Parenteral Nutrition-Associated Liver Disease: Increased risk in patients who received parenteral nutrition for greater than 2 weeks, especially preterm neonates. Monitor liver tests; if abnormalities occur consider discontinuation or dosage reduction.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, Hypertriglyceridemia, and Essential Fatty Acid Deficiency: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates.
Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting, and hyperglycemia. Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, and nosocomial infection.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use SMOFlipid safely and effectively. Please see full prescribing information for SMOFlipid (lipid injectable emulsion), for intravenous use at www.FreseniusKabiNutrition.com/SMOFlipidPI.
INDICATIONS AND USAGE
Intralipid is indicated as a source of calories and essential fatty acids for patients requiring parenteral nutrition (PN) and as a source of essential fatty acids for prevention of essential fatty acid deficiency (EFAD).
IMPORTANT SAFETY INFORMATION
Intralipid 20% Pharmacy Bulk Package (lipid injectable emulsion), for intravenous use and Intralipid 30% Pharmacy Bulk Package (lipid injectable emulsion), for intravenous use are for admixing use only and are not intended for direct intravenous administration.
Intralipid 30% (lipid injectable emulsion) Pharmacy Bulk Package must be combined with other PN fluids. Diluting Intralipid 30% with an intravenous fluid such as normal saline or other diluent does not produce a dilution that is equivalent in composition to Intralipid 10% or 20% intravenous lipid emulsions. Therefore, diluents other than dextrose and amino acids should not be used to prepare admixtures for direct intravenous administration. When Intralipid 30% is diluted, strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.125 g/kg/hour for neonates and infants.
Recommended dosage depends on age, energy expenditure, clinical status, body weight, tolerance, ability to metabolize and eliminate lipids, and consideration of additional energy given to the patient. Protect the admixed PN solution from light. Use a 1.2 micron in-line filter during administration.
Dosage for Intralipid 20%

Intralipid is contraindicated in patients with:
- Known hypersensitivity to egg, soybean, or peanut, or any of the active ingredients or excipients
- Severe disorders of lipid metabolism characterized by hypertriglyceridemia (serum triglyceride > 1,000 mg/dL)
Risk of Clinical Decompensation with Rapid Infusion of Intravenous Lipid Emulsion in Neonates and Infants: Acute respiratory distress, metabolic acidosis, and death after rapid infusion of intravenous lipid emulsions have been reported. When Intralipid 30% is diluted, strictly adhere to the recommended total daily dosage; the hourly infusion rate should not exceed 0.125 g/kg/hour for neonates and infants.
Risk of Parenteral Nutrition-Associated Liver Disease (PNALD): Increased risk in patients who receive PN for extended periods of time, especially preterm neonates. Monitor liver function tests; if abnormalities occur consider discontinuation or dosage reduction.
Hypersensitivity Reactions: Monitor for signs or symptoms. Discontinue infusion if reactions occur.
Risk of Infections, Fat Overload Syndrome, Refeeding Syndrome, and Hypertriglyceridemia: Monitor for signs and symptoms; monitor laboratory parameters.
Aluminum Toxicity: Increased risk in patients with renal impairment, including preterm neonates.
Most common adverse drug reactions (≥5%) from clinical trials in adults were nausea, vomiting, and pyrexia. Most common adverse drug reactions (≥5%) from clinical trials in pediatric patients were anemia, vomiting, increased gamma-glutamyltransferase, and cholestasis.
Vitamin K Antagonists (e.g., warfarin): Anticoagulant activity may be counteracted; increase monitoring of coagulation parameters.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
This Important Safety Information does not include all the information needed to use Intralipid safely and effectively. Please see full prescribing information, for intravenous use at www.FreseniusKabiNutrition.com/Intralipid20PI and www.FreseniusKabiNutrition.com/Intralipid30PI.